5968-11-6
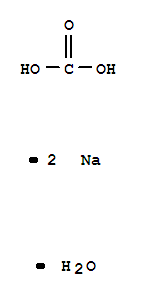
- Product Name:Sodium carbonate, monohydrate
- Molecular Formula:CH2Na2O4
- Purity:99%
- Molecular Weight:124.0037
Product Details;
CasNo: 5968-11-6
Molecular Formula: CH2Na2O4
Appearance: white powder
Quality Manufacturer Supply High Purity 99% Sodium carbonate, monohydrate 5968-11-6 with Reasonable Price
- Molecular Formula:CH2Na2O4
- Molecular Weight:124.0037
- Appearance/Colour:white powder
- Melting Point:851 °C(lit.)
- Boiling Point:333.6 °C at 760 mmHg
- Flash Point:169.8 °C
- PSA:72.42000
- Density:2.53 g/cm3
- LogP:-2.51130
Sodium carbonate, monohydrate(Cas 5968-11-6) Usage
|
Production Methods |
Sodium carbonate is produced by the ammonia-soda process, also known as the Solvay process. |
|
Pharmaceutical Applications |
Sodium carbonate is used as an alkalizing agent in injectable, ophthalmic, oral, and rectal formulations. In effervescent tablets or granules, sodium carbonate is used in combination with an acid, typically citric acid or tartaric acid. When the tablets or granules come into contact with water, an acid– base reaction occurs in which carbon dioxide gas is produced and the product disintegrates. Raw materials with low moisture contents are required to prevent the early triggering of the effervescent reaction. As an alkalizing agent, concentrations of sodium carbonate between 2% and 5% w/w are used in compressed tablet formulations. As an effervescent agent, concentrations of sodium carbonate up to 10% w/w can be used. Therapeutically, sodium carbonate is also used as an oral antacid. |
|
Safety |
Sodium carbonate is used in injectable, oral, and rectal pharmaceutical formulations. The pure form of sodium carbonate is mildly toxic by ingestion, moderately toxic by inhalation and SC routes, and very toxic by the IP route. It is irritating to the skin and eyes. Dust and vapors of sodium carbonate may irritate mucous membranes, causing coughing and shortness of breath. It also has experimental reproductive effects. Sodium carbonate can migrate to food from packaging materials. When used as an excipient or antacid, sodium carbonate is generally regarded as a nontoxic and nonirritating material. LD50 (mouse, IP): 0.12 g/kg LD50 (mouse, SC): 2.21 g/kg LD50 (rat, oral): 4.09 g/kg |
|
storage |
Sodium carbonate converts to the monohydrate form when in contact with water and produces heat. It begins to lose carbon dioxide at temperatures above 400℃ and decomposes before boiling. Store in airtight containers. |
|
Incompatibilities |
Sodium carbonate decomposes when in contact with acids in the presence of water to produce carbon dioxide and effervescence. It may react violently with aluminum, phosphorous pentoxide, sulfuric acid, fluorine, and lithium. |
|
Regulatory Status |
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (injections; ophthalmic solution; oral capsules and tablets; rectal suspensions). Included in the Canadian List of Acceptable Non-medicinal Ingredients. Included in parenteral (powder for solution for injection) and nonparenteral medicines (oral effervescent tablets, soluble tablets, granules, lozenges, chewing gums) licensed in the UK. USP32–NF27 allows either the anhydrous or the monohydrate form. |
|
Chemical Composition and Structure |
Sodium carbonate monohydrate consists of sodium ions (Na+), carbonate ions (CO3^2-), and water molecules. |
|
Analysis Method |
The kinetics and dynamics of sodium carbonate monohydrate reactions can be analyzed using techniques such as thermogravimetric methods, terahertz spectroscopy, and solid-state analytical techniques like scanning electron microscopy and X-ray powder diffraction.[3] |
|
Industrial uses |
The most prominent characteristic of soda ash in solution is the high buffered pH response. For example, changing the sodium carbonate dosage from 0.03% to 30% only causes the solution pH to change from 10.5 to 11.7. Because of this, in mineral processing application, soda ash is used for pH control to a maximum value of 10.5. |
InChI:InChI=1/CH2O3.Na.H2O/c2-1(3)4;;/h(H2,2,3,4);;1H2/q;+1;/p-2
Relevant Products
-
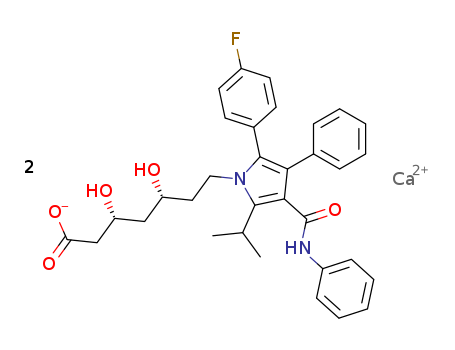
Atorvastatin calcium
CAS:134523-03-8
-
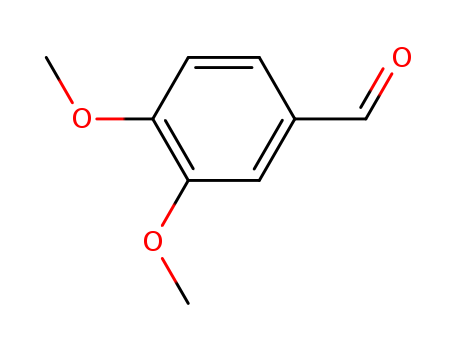
Veratraldehyde
CAS:120-14-9
-
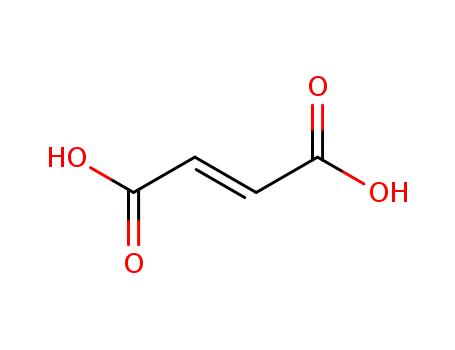
Fumaric acid
CAS:110-17-8