134523-03-8
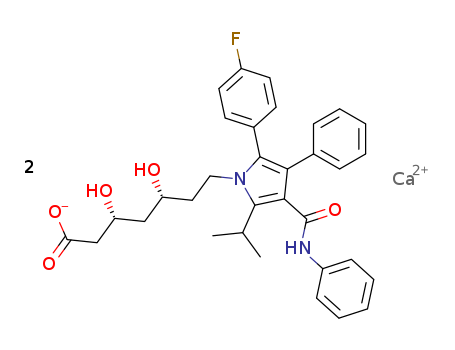
- Product Name:Atorvastatin calcium
- Molecular Formula:C66H68CaF2N4O10
- Purity:99%
- Molecular Weight:1155.36
Product Details;
CasNo: 134523-03-8
Molecular Formula: C66H68CaF2N4O10
Appearance: White Crystalline Powder
Quality Manufacturer Supply High Purity 99% Atorvastatin calcium 134523-03-8 with Reasonable Price
- Molecular Formula:C66H68CaF2N4O10
- Molecular Weight:1155.36
- Appearance/Colour:White Crystalline Powder
- Melting Point:176-178 °C
- Boiling Point:722.2 °C at 760 mmHg
- Flash Point:390.6 °C
- PSA:229.24000
- LogP:10.10380
Atorvastatin calcium(Cas 134523-03-8) Usage
|
Indication and application |
LIPITOR is mainly indicated for the treatment of cardiovascular disease and dyslipidemia due to its effect in lowering the cholesterol in the blood[7-9]. LIPITOR is a prescription medicine that lowers cholesterol in the blood. It lowers the LDL-C["bad” cholesterol] and triglycerides in your blood. It can raise your HDL-C["good" cholesterol] as well[10]. LIPITOR is for adults and children over 10 whose cholesterol does not come down enough with exercise and a low-fat diet alone. LIPITOR can lower the risk for heart attack, stroke, certain types of heart surgery, and chest pain in patients who have heart disease or risk factors for heart disease such as age, smoking, high blood pressure, low HDL-C, or heart disease in the family. LIPITOR can lower the risk for heart attack or stroke in patients with diabetes and risk factors such as eye problems, kidney problems, smoking, or high blood pressure[7-10]. As a drug for the treatment of cardiovascular, LIPITOR is indicated to reduce the risk of myocardial infarction, reduce the risk of stroke and reduce the risk for revascularization procedures and angina[7, 8, 10, 11]. Moreover, for adults patients with type II diabetes[having multiple risk factors for coronary heart disease such as retinopathy, albuminuria, smoking, or hypertension], it is also highly effective[12, 13]. For adults diagnosed of clinically evident coronary heart disease, LIPITOR can reduce the risk of non-fatalmyocardial infarction, fatal and non-fatal stroke, revascularization procedures as well as hospitalization for CHF and angina. As a drug for the treatment of hyperlipidemia, it is used as an adjunct to diet to reduce elevated total-C, LDL-C, apo B, and TGlevels and to increase HDL-C in adult patients with primary hypercholesterolemia or in pediatric patients as well as for the treatment of adult patients with elevated serum TG levels[7, 8, 14]. |
|
Mode of action |
Atorvastatin takes effect through selectively and competitively inhibiting the hepatic enzyme HMG-CoA reductase, which is responsible for converting HMG-CoA to mevalonate in the cholesterol biosynthesis pathway[15, 16]. This results in a subsequent decrease in hepatic cholesterol levels. Decreased hepatic cholesterol levels stimulates upregulation of hepatic LDL-C receptors which increases hepatic uptake of LDL-C and reduces serum LDL-C concentrations[9. 16]. |
|
Adverse reactions |
The most serious adverse reactions associated with LIPITOR include Rhabdomyolysis and myopathy as well as liver enzyme abnormality[18]. Common side effects include headache, hoarseness, lower back or side pain, pain or tenderness around the eyes and cheekbones, painful or difficult urination, stuffy or runny nose[8]. Some less common side effects also include abdominal or stomach pain, back pain, belching or excessive gas, constipation, general feeling of discomfort or illness, heartburn, indigestion or stomach discomfort, lack or loss of strength, loss of appetite, nausea, shivering, sweating, trouble sleeping and vomiting[8]. |
|
Warning and precaution |
Pregnant or lactation women should be disabled from using LIPITOR. Serious drug interactions can occur when certain medicines are used together with atorvastatin. So you should provide those information to your doctor before taking LIPITOR[7, 8, 17]. In rare cases, LIPITOR can cause a condition that results in the breakdown of skeletal muscle tissue, leading to kidney failure. Call your doctor right away if you have unexplained muscle pain, tenderness, or weakness especially if you also have fever, unusual tiredness, and dark colored urine. Avoid eating foods that are high in fat or cholesterol. LIPITOR will not be as effective in lowering your cholesterol if you do not follow a cholesterol-lowering diet plan[7, 17]. LIPITOR is not approved for use by anyone younger than 10 years old and those patients who are allergic to it, or of liver disease. Moreover, since LIPITOR may pass into breast milk and could harm a nursing baby. Do not breast-feed while you are taking this medicine[7, 17]. Patients who have a history of liver problems, muscle pain or weakness, kidney disease, diabetes. a thyroid disorder; or drink more than 2 alcoholic beverages daily should take with care[7, 17]. |
|
Reference |
https://www.lipitor.com Kokilambigai, K. S., R. Seetharaman, and K. S. Lakshmi. "Critical Review on the Analytical Techniques for the Determination of the Oldest Statin—Atorvastatin—in Bulk, Pharmaceutical Formulations and Biological Fluids." Critical Reviews in Analytical Chemistry 47.6(2017]:538. Teckchandani, S., et al. "Rhabdomyolysis following co-prescription of Fusidic Acid and Atorvastatin, with review of Statin Antimicrobial Drug Interactions." Scottish Medical Journal 54.3(2009]:50-50. Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, et al.[September 2016]. "Interpretation of the evidence for the efficacy and safety of statin therapy". Lancet. 388: 2532–2561. http://www.crainsnewyork.com/article/20111228/HEALTH_CARE/111229902 Maggon K: Best-selling human medicines 2002-2004. Drug Discov Today. 2005 Jun 1;10(11]:739-42. https://www.webmd.com/drugs/2/drug-3330/lipitor-oral/details https://www.drugs.com/monograph/atorvastatin-calcium.html https://www.drugbank.ca/drugs/DB01076 Jukema, J. W., et al. "LDL-C/HDL-C ratio in subjects with cardiovascular disease and a low HDL-C: results of the RADAR[Rosuvastatin and Atorvastatin in different Dosages And Reverse cholesterol transport] study. " Current Medical Research & Opinion 21.11(2005]:1865-1874. Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, Davignon J, Erbel R, Fruchart JC, Tardif JC, Schoenhagen P, Crowe T, Cain V, Wolski K, Goormastic M, Tuzcu EM[April 2006]. "Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial". JAMA.? Chaturvedi, S., Zivin, J., Breazna, A., Amarenco, P., Callahan, A., & Goldstein, L. B., et al.[2009]. Atorvastatin, stroke, transient ischemic attack. Neurology, 72(8], 818-819. Colhoun, H. M., et al. "Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study[CARDS]: multicentre randomised placebo-controlled trial. " Lancet364.9435(2004]:685-696. Milionis, H., et al. "Th-P16:381 Treating to target patients with primary hyperlipidemia: Comparison of the effects of atorvastatin and rosuvastatin[The atoros study]." Current Medical Research & Opinion22.6(2006]:1123-1131. Youssef, S, et al. "The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. " Nature 420.6911(2002]:78-84. Black, D. M., R. G. Bakkerarkema, and J. W. Nawrocki. "An overview of the clinical safety profile of atorvastatin[lipitor], a new HMG-CoA reductase inhibitor. " Archives of Internal Medicine 158.6(1998]:577. https://www.rxlist.com/lipitor-drug.htm#side_effects_interactions |
|
Biological Activity |
Potent HMG-CoA reductase inhibitor (IC 50 = 8 nM). Reduces circulating LDL-C by inhibiting cholesterol biosynthesis and inducing expression of LDL receptors. Inhibits smooth muscle cell proliferation in vitro and exhibits antinociceptive effects in the inflammatory hypernociception model. |
|
Chemical Composition and Structure |
Atorvastatin calcium is an organic calcium salt composed of calcium cations and atorvastatin anions in a 1:2 ratio. It is a member of the lipid-lowering agents and a significant inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, which catalyzes the conversion of HMG-CoA to mevalonate, an early step in cholesterol synthesis. |
|
Formulation and Bioavailability Enhancement |
Atorvastatin calcium has limited bioavailability when taken orally due to its low solubility in low pH and extensive first-pass effect. Different formulation strategies have been utilized to overcome these obstacles, such as solid dispersion, co-crystallization, and incorporation of self-emulsifying vehicles. |
|
Co-crystallization for Solubility Enhancement |
Co-crystallization is a method used to enhance the solubility of hydrophobic drugs like atorvastatin calcium. Co-crystals of atorvastatin calcium have been prepared using various co-formers such as citric acid and nicotinamide to enhance solubility. Coamorphous solid forms of atorvastatin calcium with conformers like isonicotinamide (INA) and maleic acid (MA) have also been prepared using spray drying techniques, resulting in improved solubility properties compared to the crystalline form. |
|
Pleiotropic Effects |
Atorvastatin calcium exhibits pleiotropic effects as an anti-inflammatory drug in addition to its main antihyperlipidemic action. These pleiotropic effects contribute to the therapeutic benefits of atorvastatin calcium beyond its cholesterol-lowering properties. |
|
Pharmacological Action and Clinical Use |
Atorvastatin calcium is a statin drug used to lower cholesterol levels effectively. It is prescribed to reduce mortality risks in patients with cardiovascular disease by effectively lowering blood cholesterol levels. Atorvastatin calcium has been shown to effectively reduce both cholesterol and triglycerides in patients. |
|
Overview |
Atorvastatin calcium[trade name: Lipitor] is a statin-class medication used mainly for lowering the lipid as well as preventing the event associated with cardiovascular disease[1, 2]. Being similar to other kinds of statins, atorvastatin take effects by inhibiting HMG-CoA reductase, an enzyme found in liver tissue that plays a key role in production of cholesterol in the body. Atorvastatin acts primarily in the liver. Decreased hepatic cholesterol levels further increases hepatic uptake of cholesterol and reduces plasma cholesterol levels[3, 4]. Lipitor, since 1996, has become the world's best-selling medication to that point[5, 6], with more than US$125 billion in sales over approximately 14.5 years. As of 2016, in the UK, atorvastatin costs about £2 per month[5]. |
|
Definition |
ChEBI: An organic calcium salt composed of calcium cations and atorvastatin anions in a 1:2 ratio. |
InChI:InChI=1/2C33H35FN2O5.2Ca.2H/c2*1-21(2)31-30(33(41)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-26(37)19-27(38)20-28(39)40;;;;/h2*3-16,21,26-27,37-38H,17-20H2,1-2H3,(H,35,41)(H,39,40);;;;/q;;;+2;;/p-2/t2*26-,27-;;;;/m11..../s1/r2C33H35FN2O5.CaH2.Ca/c2*1-21(2)31-30(33(41)35-25-11-7-4-8-12-25)29(22-9-5-3-6-10-22)32(23-13-15-24(34)16-14-23)36(31)18-17-26(37)19-27(38)20-28(39)40;;/h2*3-16,21,26-27,37-38H,17-20H2,1-2H3,(H,35,41)(H,39,40);1H2;/q;;;+2/p-2/t2*26-,27-;;/m11../s1
134523-03-8 Relevant articles
Method for purifying atorvastatin calcium intermediate
-
Paragraph 0031-0032; 0035-0043; 0045-0046; 0049; 0051;..., (2021/09/01)
The atorvastatin calcium intermediate is...
PROCESS FOR THE CONTINUOUS MANUFACTURE OF STATINS
-
Page/Page column 30-31, (2021/06/04)
The present invention relates to process...
Asymmetric synthesis of (-)-atorvastatin calcium by tandem catalysis
Fuwa, Haruhiko,Minami, Riko,Murata, Keisuke
, p. 2028 - 2035 (2021/09/16)
A seven-step synthesis of (-)-atorvastat...
Preparation method of crystal form I atorvastatin calcium
-
Paragraph 0043-0068, (2020/02/29)
The invention discloses a preparation me...
134523-03-8 Process route
-
-
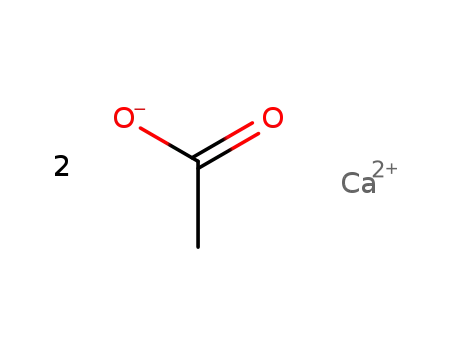
62-54-4
calcium acetate

-
-
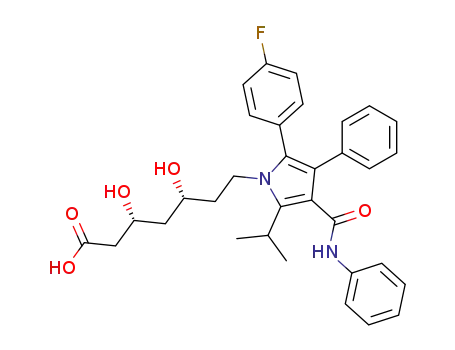
134523-00-5,110862-48-1
atorvastatin

-
-
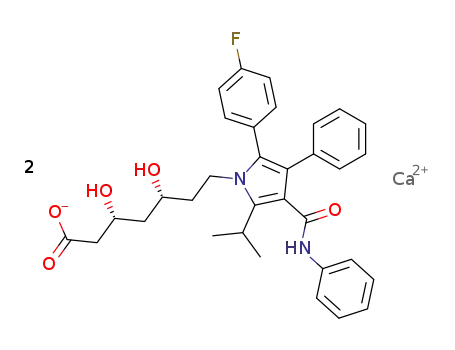
134523-03-8
lipitor
| Conditions | Yield |
|---|---|
|
calcium acetate; atorvastatin;
In
water; acetonitrile;
at 44 ℃;
for 1.25h;
With
sodium hydroxide;
In
water;
at 30 - 70 ℃;
for 9.5h;
Product distribution / selectivity;
|
84.35% |
-
-
C49H51FN2O5Si

-

-
134523-03-8
lipitor
| Conditions | Yield |
|---|---|
|
With
calcium hydroxide;
In
ethanol; water;
at 70 ℃;
for 5.5h;
|
26% |
134523-03-8 Upstream products
-
62-54-4

calcium acetate
-
134523-00-5

atorvastatin
-
125971-95-1
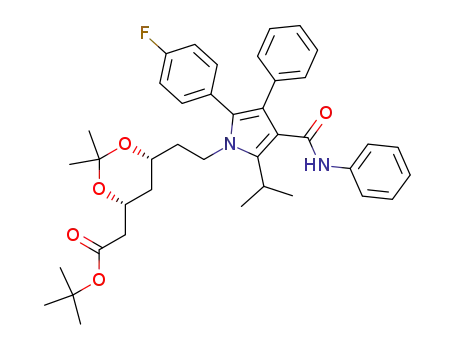
tert-butyl (4R,6R)-6-{2-[2-(4-fluorophenyl)-5-isopropyl-3-phenyl-4-(phenylcarbamoyl)pyrrol-1-yl]ethyl}-2,2-dimethyl-1,3-dioxane-4-acetate
-
134523-01-6
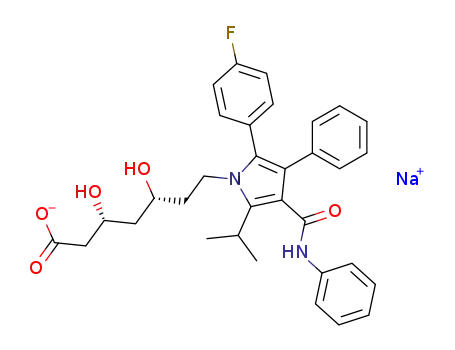
atorvastatin sodium
134523-03-8 Downstream products
-
134523-01-6

atorvastatin sodium
-
134523-00-5

atorvastatin
-
873950-19-7
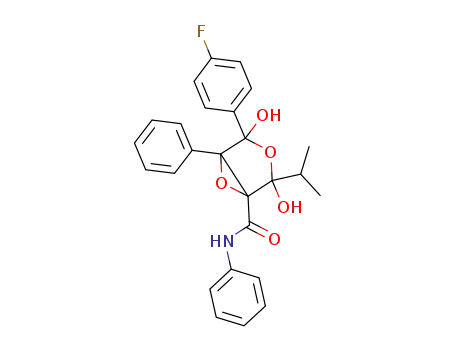
4-(4-fluoro-phenyl)-2,4-dihydroxy-2-isopropyl-5-phenyl-3,6-dioxa-bicyclo[3.1.0]hexane-1-carboxylic acid phenylamide
-
873950-18-6
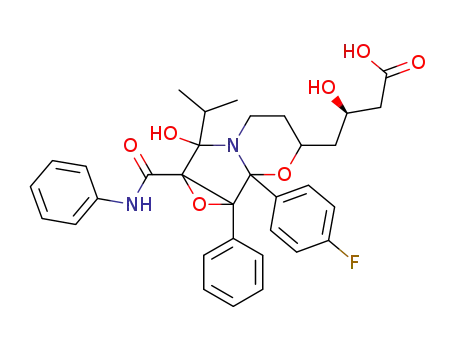
4-[1b-(4-fluoro-phenyl)-6-hydroxy-6-isopropyl-1a-phenyl-6a-phenylcarbamoyl-hexahydro-1,2-dioxa-5a-aza-cyclopropa[a]inden-3-yl]-3-(R)-hydroxy-butyric acid
Relevant Products
-
Sodium carbonate, monohydrate
CAS:5968-11-6
-
Magnesium sulfate
CAS:7487-88-9
-
Sodium alginate
CAS:9005-38-3