110-17-8
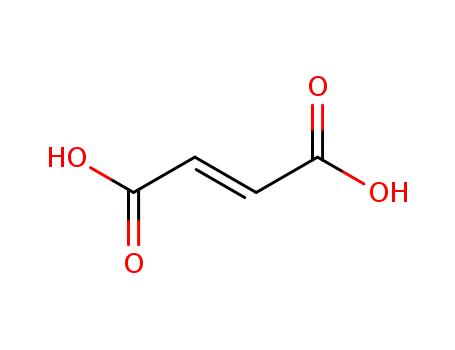
- Product Name:Fumaric acid
- Molecular Formula:C4H4O4
- Purity:99%
- Molecular Weight:116.073
Product Details;
CasNo: 110-17-8
Molecular Formula: C4H4O4
Appearance: white powder or colourless crystals
Factory Sells Best Quality Fumaric acid 110-17-8 with GMP standards
- Molecular Formula:C4H4O4
- Molecular Weight:116.073
- Appearance/Colour:white powder or colourless crystals
- Melting Point:295-300 ºC
- Boiling Point:355.5°Cat760mmHg
- Flash Point:230 ºC
- PSA:74.60000
- Density:1.499 g/cm3
- LogP:-0.28820
Fumaric acid(Cas 110-17-8) Usage
|
Chemical Description |
Fumaric acid is used to transform the bases into salts. |
|
Use Description |
Fumaric acid, a specific chemical compound, serves diverse roles in various fields. In the food industry, it acts as a food additive and acidulant, enhancing the flavor and preserving the quality of various products. In the pharmaceutical sector, it plays a crucial role as an ingredient in the formulation of medications, particularly in controlled-release drug delivery systems. Moreover, in the field of polymer science, fumaric acid is used in the production of biodegradable plastics and resins, contributing to the development of more sustainable materials. Its applications in food, pharmaceuticals, and polymers underscore its significance in improving taste, enabling drug delivery, and promoting environmental sustainability within these distinct fields. |
|
Industrial Applications and Production |
Fumaric acid, also known as trans-1,2-ethylenedicarboxylic acid, is extensively used in various industries including food, chemicals, agriculture, and pharmaceuticals. Its multifunctional structure, featuring a carbon-carbon double bond and two carboxylic acid groups, allows for easy esterification and polymerization, making it a key chemical feedstock for producing paper resins, unsaturated polyester resins, alkyd resins, plasticizers, and other industrial products. Additionally, its non-toxic nature and unique flavor have led to its widespread use as a food acidulant and beverage ingredient. |
|
Synthesis Methods |
Fumaric acid is primarily synthesized through the isomerization of maleic acid, typically catalyzed by mineral acids, peroxy compounds, or thiourea. This petrochemical method, while yielding high production rates, often involves high temperatures, leading to the formation of by-products and significant energy consumption. Alternatively, microbial fermentation processes have gained attention due to their sustainability and environmentally friendly characteristics, offering a potential alternative to petrochemical synthesis. |
|
Uses in Various Industries |
Fumaric acid finds applications in diverse industries, including food, medicine, and construction. It is used for native chitosan modification and as an active material in antioxidant, antibacterial, and antiviral reagents. It is also recognized as one of the "top 12" chemical building blocks due to its wide-ranging applications and importance in industrial processes. |
|
Biological Functions |
Fumaric acid is a component of the tricarboxylic acid (TCA) cycle and plays a role in metabolic pathways in various organisms. Some lactobacilli utilize the TCA cycle in the direction of reductive reactions, metabolizing fumarate to malic acid or succinic acid. Additionally, fumaric acid and its salts are commonly used in terrestrial animal feeds. |
|
Use in Wine Production |
Fumaric acid is permitted as an additive for wine acidification and inhibition of malolactic fermentation (MLF). It can effectively inhibit MLF in red wines at specific concentrations, improving microbiological stability and freshness while allowing for the reduction of SO2 levels. Tastings have shown that fumaric acid enhances acidity and body in wines, making it a valuable technological additive in winemaking. |
InChI:InChI=1/C4H4O4/c5-3(6)1-2-4(7)8/h1-2H,(H,5,6)(H,7,8)/b2-1+
110-17-8 Relevant articles
Production of Plant Phthalate and its Hydrogenated Derivative from Bio-Based Platform Chemicals
Lu, Rui,Lu, Fang,Si, Xiaoqin,Jiang, Huifang,Huang, Qianqian,Yu, Weiqiang,Kong, Xiangtao,Xu, Jie
, p. 1621 - 1627 (2018)
Direct transformation of bio-based platf...
-
Rozelle,Alberty
, p. 1637 (1957)
-
Some aspects of the Knoevenagel-Doebner and Wittig reactions
Aparicio, F. J. Lopez,Herrera, F. J. Lopez
, p. C4 - C7 (1980)
-
An efficient and practical system for the catalytic oxidation of alcohols, aldehydes, and α,β-unsaturated carboxylic acids
Grill, Joseph M.,Ogle, James W.,Miller, Stephen A.
, p. 9291 - 9296 (2006)
(Chemical Equation Presented) Upon expos...
TAN-1323 C and D, new concanamycin-group antibiotics; Detection of the angiostatic activity with a wide range of macrolide antibiotics
Ishii,Hida,Iinuma,Muroi,Nozaki
, p. 12 - 20 (1995)
We detected potent angiostatic activity ...
-
Butkewitsch
, p. 100,104 (1927)
-
Potent covalent inhibitors of bacterial urease identified by activity-reactivity profiling
Macegoniuk, Katarzyna,Kowalczyk, Rafa?,Rudzińska, Anna,Psurski, Mateusz,Wietrzyk, Joanna,Berlicki, ?ukasz
, p. 1346 - 1350 (2017)
Covalent enzyme inhibitors constitute a ...
Purification and characterization of a lyase from the EDTA-degrading bacterial strain DSM 9103 that catalyzes the splitting of [S,S]-ethylenediaminedisuccinate, a structural isomer of EDTA
Witschel, Margarete,Egli, Thomas
, p. 419 - 428 (1997)
The bacterial strain DSM 9103, able to u...
Structural and kinetic studies on adenylosuccinate lyase from Mycobacterium smegmatis and Mycobacterium tuberculosis provide new insights on the catalytic residues of the enzyme
Banerjee, Sanchari,Agrawal, Monika J.,Mishra, Diptimayee,Sharan, Siddharth,Balaram, Hemalatha,Savithri, Handanhal S.,Murthy, Mathur R. N.
, p. 1642 - 1658 (2014)
Adenylosuccinate lyase (ASL), an enzyme ...
Microflow photochemistry - A reactor comparison study using the photochemical synthesis of terebic acid as a model reaction
Aida, Shin,Terao, Kimitada,Nishiyama, Yasuhiro,Kakiuchi, Kiyomi,Oelgem?ller, Michael
, p. 5578 - 5581 (2012)
The continuous-microflow photochemical s...
Mechanism of the Enzymic Elimination of Ammonia from 3-Substituted Aspartic Acids by 3-Methylaspartase
Botting, Nigel P.,Akhtar, Mahmoud,Cohen, Mark A.,Gani, David
, p. 1371 - 1373 (1987)
Kinetic experiments with 3-methylasparta...
The 3-methylaspartase reaction probed using 2H- and 15N-Isotope effects for three substrates: A flip from a concerted to a carbocationic amino-enzyme elimination mechanism upon changing the C-3 stereochemistry in the substrate from R to S
Gani, David,Archer, Catherine H.,Botting, Nigel P.,Pollard, John R.
, p. 977 - 990 (1999)
The mechanisms of the elimination of amm...
ELIMINATION OF HYDROGEN FLUORIDE FROM FLUORINATED SUCCINIC ACIDS.(II) KINETICS OF DEHYDROFLUORINATION OF FLUORO-, 2,2-DIFLUORO-, MESO- AND DL-2,3-DIFLUORO-, AND TRIFLUOROSUCCINIC ACIDS
Hudlicky, M.,Glass, T. E.
, p. 15 - 28 (1983)
Elimination of hydrogen fluoride from fl...
-
Herasymenko,Tyvonuk
, p. 78 (1930)
-
A new dipeptide antibiotic from Streptomyces collinus, Lindenbein.
Molloy,Lively,Gale,Gorman,Boeck
, p. 137 - 140 (1972)
-
On chemical reactions in the laser-induced breakdown of a liquid
Margulis,Ovchinnikov,Margulis
, p. 986 - 990 (2006)
It is shown experimentally that a laser-...
Asymmetric Synthesis of (S)- and (R)-Malic Acid from Ketene and Chloral
Wynberg, Hans,Staring, Emiel G. J.
, p. 166 - 168 (1982)
Quinidine (5) catalyzes the addition of ...
N-terminal truncation of a maleate cis-trans isomerase from Rhodococcus jostii RHA1 results in a highly active enzyme for the biocatalytic production of fumaric acid
Liu, Xiangtao,Zhao, Qing,Ren, Jie,Dong, Wenyue,Wu, Qiaqing,Zhu, Dunming
, p. 44 - 50 (2013)
As part of the project to develop an eff...
Mechanism of 3-methylaspartase probed using deuterium and solvent isotope effects and active-site directed reagents: Identification of an essential cysteine residue
Pollard, John R.,Richardson, Susan,Akhtar, Mahmoud,Lasry, Philippe,Neal, Tracy,Botting, Nigel P.,Gani, David
, p. 949 - 975 (1999)
The mechanism of the l-threo-3-methylasp...
-
Ssadikow
, p. 504,508 (1923)
-
Poly (4-vinylpyridine) catalyzed isomerization of maleic acid to fumaric acid
Li, Qiang,Tao, Weihua,Li, Aimin,Zhou, Qing,Shuang, Chendong
, p. 148 - 153 (2014)
Fumaric acid is an important industrial ...
Study on the Isomerization of Maleic Acid to Fumaric Acid without Catalyst
Gao, Zhuo,Chen, Wangmi,Chen, Xiaoting,Wang, Dali,Yi, Shouzhi
, p. 920 - 924 (2018)
Fumaric acid is an important food additi...
The Structure of Chalybaeizanic Acid and Quaesitic Acid, Two New Lichen Depsidones Related to Salazinic Acid
Elix, John A.,Wardlaw, Judith H.
, p. 713 - 716 (1999)
The depsidones chalybaeizanic acid (1,4,...
-
Ikutani,Y.
, p. 3602 - 3603 (1970)
-
-
Tilden,Marshall
, p. 494 (1895)
-
Oxidation of aliphatic side chains in anthracene Diels-Alder adducts
McCormick, Frankie A.,Marquardt, Donald J.
, p. 5169 - 5172 (1994)
An efficient oxidation of methyl and pri...
A covalent succinylcysteine-like intermediate in the enzyme-catalyzed transformation of maleate to fumarate by maleate isomerase
Fisch, Florian,Fleites, Carlos Martinez,Delenne, Marie,Baudendistel, Nina,Hauer, Bernhard,Turkenburg, Johan P.,Hart, Sam,Bruce, Neil C.,Grogan, Gideon
, p. 11455 - 11457 (2010)
Maleate isomerase (MI), a member of the ...
Purification and characterization of fumarase from Corynebacterium glutamicum
Genda, Tomoko,Watabe, Shoji,Ozaki, Hachiro
, p. 1102 - 1109 (2006)
Fumarase (EC 4.2.1.2) from Corynebacteri...
-
Davies,Evans
, p. 74,77 (1956)
-
-
Taube, H.
, p. 526 - 531 (1943)
-
Pompon Dahlia-like Cu2O/rGO Nanostructures for Visible Light Photocatalytic H2 Production and 4-Chlorophenol Degradation
Karthikeyan, Sekar,Ahmed, Kassam,Osatiashtiani, Amin,Lee, Adam F.,Wilson, Karen,Sasaki, Keiko,Coulson, Ben,Swansborough-Aston, Will,Douthwaite, Richard E.,Li, Wei
, p. 1699 - 1709 (2020)
Hierarchical Cu2O nanospheres with a Pom...
-
Owen,Simonsen
, (1933)
-
Characterization of the cross-linked structure of fumarate-based degradable polymer networks
Timmer, Mark D.,Jo, Seongbong,Wang, Chuanyue,Ambrose, Catherine G.,Mikos, Antonios G.
, p. 4373 - 4379 (2002)
A new method was developed to examine ne...
Size-Dependent Visible Light Photocatalytic Performance of Cu2O Nanocubes
Karthikeyan, Sekar,Kumar, Santosh,Durndell, Lee J.,Isaacs, Mark A.,Parlett, Christopher M. A.,Coulson, Ben,Douthwaite, Richard E.,Jiang, Zhi,Wilson, Karen,Lee, Adam F.
, p. 3554 - 3563 (2018)
Well-defined Cu2O nanocubes with tunable...
Ionic liquids breakdown by Fenton oxidation
Munoz, Macarena,Domínguez, Carmen M.,De Pedro, Zahara M.,Quintanilla, Asunción,Casas, Jose A.,Rodriguez, Juan J.
, p. 16 - 21 (2015)
Fenton oxidation has proved to be an eff...
-
Gelles,Pitzer
, p. 1974,1975, 1976 (1955)
-
Ordered mesoporous carbon as an efficient heterogeneous catalyst to activate peroxydisulfate for degradation of sulfadiazine
Cao, Di,Chen, Fan,Cheng, Hao,Huang, Cong,Li, Zhi-Ling,Liang, Bin,Nan, Jun,Sun, Kai,Wang, Ai-Jie
supporting information, (2022/01/26)
Catalytic potential of carbon nanomateri...
Preparation method of succinic acid
-
Paragraph 0028-0076, (2021/06/22)
The invention discloses a preparation me...
Biosynthesis ofl-alanine fromcis-butenedioic anhydride catalyzed by a triple-enzyme cascadeviaa genetically modified strain
Cui, Ruizhi,Liu, Zhongmei,Yu, Puyi,Zhou, Li,Zhou, Zhemin
, p. 7290 - 7298 (2021/09/28)
In industry,l-alanine is biosynthesized ...
Preparation method of fumaric acid
-
Paragraph 0031-0095, (2021/06/22)
The invention discloses a preparation me...
110-17-8 Process route
-
-
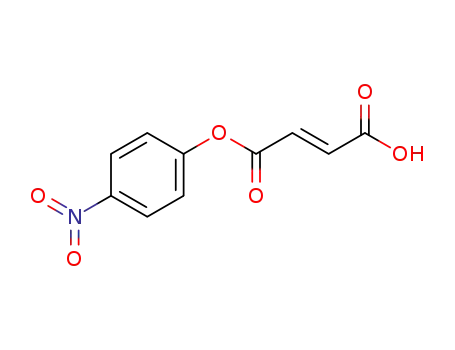
(E)-4-(4-nitrophenoxy)-4-oxobut-2-enoic acid

-
-
100-02-7,78813-13-5,89830-32-0
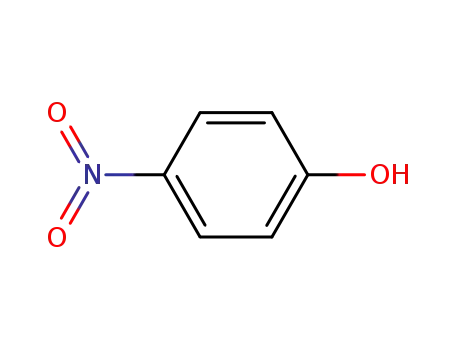
4-nitro-phenol

-
-
110-17-8,26099-09-2
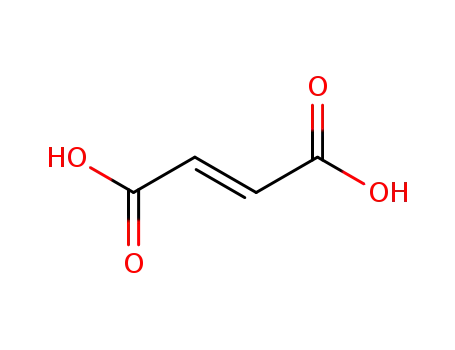
(2E)-but-2-enedioic acid
| Conditions | Yield |
|---|---|
|
With
pH=5.1 buffer; polypeptide MN-42;
In
acetonitrile;
at 16.9 ℃;
Rate constant;
other polypetides vith var. amino acids sequences;
|
|
|
With
acetate buffer; JNIIHR polypeptide;
In
acetonitrile;
at 16.85 ℃;
pH=5.1;
Further Variations:;
Reagents;
Kinetics;
|
|
|
With
AcAPLEPEYPGDNATPEQMHQYAHQLRRYINMLCONH2;
In
acetate buffer;
at 16.85 ℃;
pH=4.1;
Further Variations:;
pH-values;
Reagents;
Kinetics;
|
-
-
7732-18-5
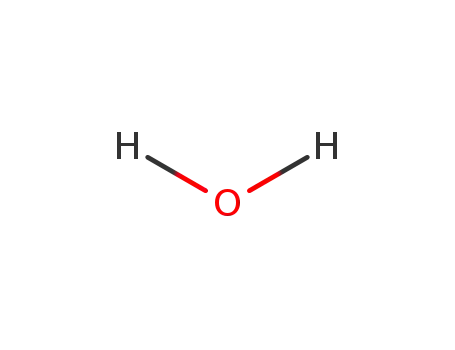
water

-
-
17356-08-0
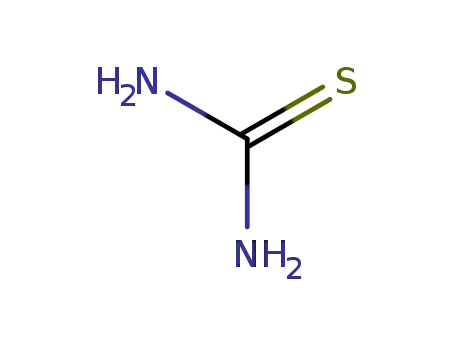
thiourea

-
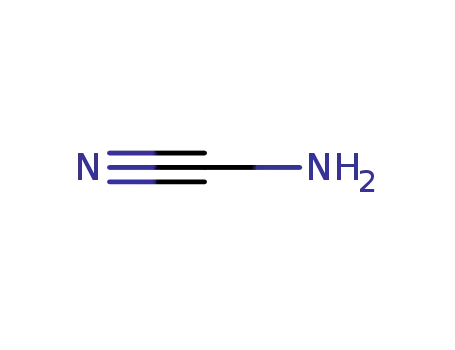
-
420-04-2
CYANAMID

-
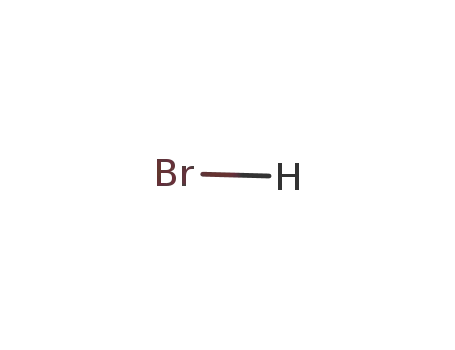
-
10035-10-6,12258-64-9
hydrogen bromide

-

-
110-17-8,26099-09-2
(2E)-but-2-enedioic acid
| Conditions | Yield |
|---|---|
|
|
110-17-8 Upstream products
-
110-89-4
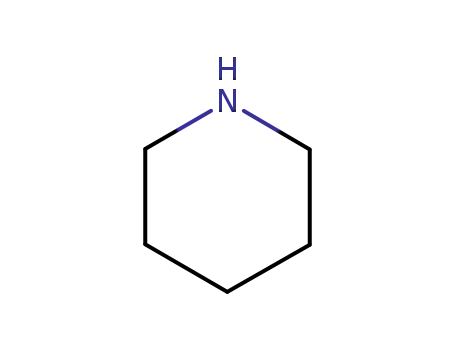
piperidine
-
110-86-1
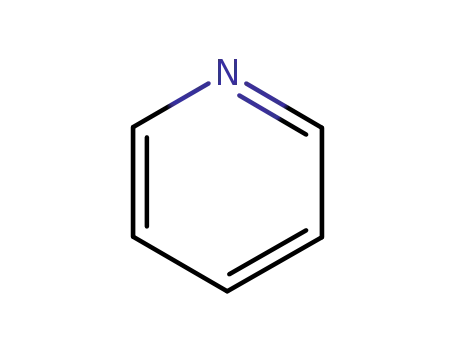
pyridine
-
141-82-2
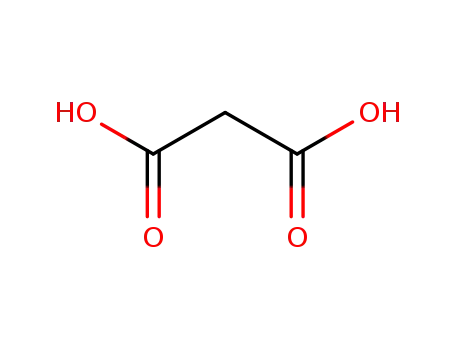
malonic acid
-
298-12-4
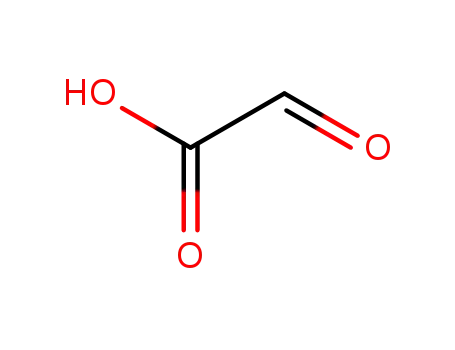
Glyoxilic acid
110-17-8 Downstream products
-
87387-81-3
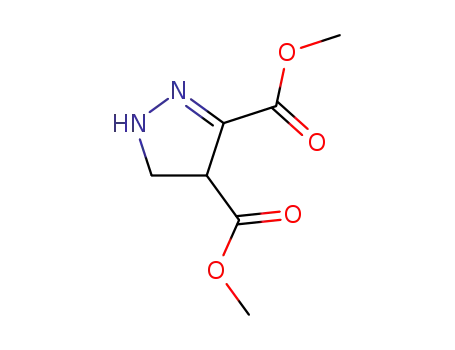
4-methoxycarbonyl-pyrazoline-3-carboxylic acid methyl ester
-
2051-95-8
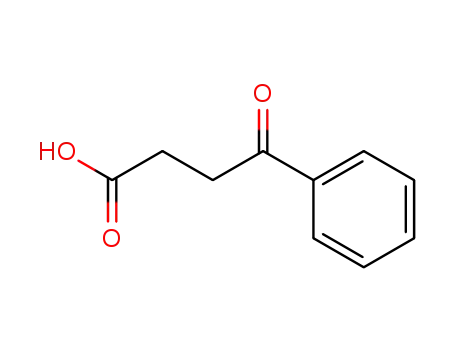
succinylbenzene
-
1148-41-0
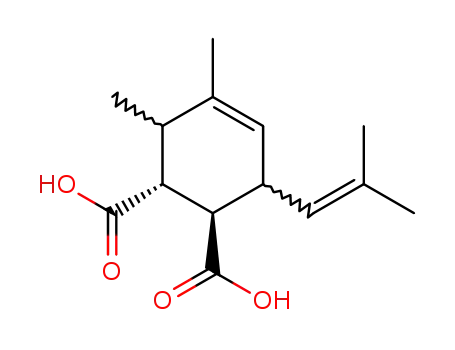
3,4-dimethyl-6-(2-methyl-propenyl)-cyclohex-4-ene-1,2-dicarboxylic acid
-
110-15-6
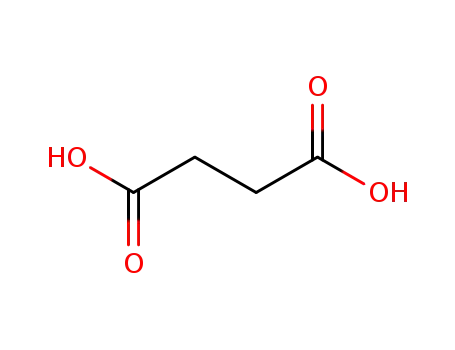
succinic acid
Relevant Products
-
Tesamorelin
CAS:218949-48-5
-
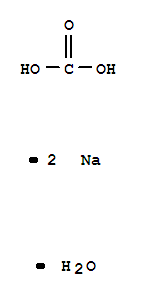
Sodium carbonate, monohydrate
CAS:5968-11-6
-
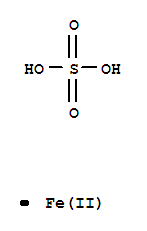
Ferrous sulfate
CAS:7720-78-7