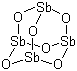
10035-04-8
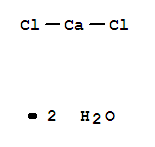
- Product Name:Calcium chloride dihydrate
- Molecular Formula:CaCl2H4O2
- Purity:99%
- Molecular Weight:147.02
Product Details;
CasNo: 10035-04-8
Molecular Formula: CaCl2H4O2
Appearance: colourless crystals or white crystalline powder
Quality Factory Sells Top Purity 99% Calcium chloride dihydrate 10035-04-8 with Safe Delivery
- Molecular Formula:CaCl2H4O2
- Molecular Weight:147.02
- Appearance/Colour:colourless crystals or white crystalline powder
- Vapor Pressure:0.01 mm Hg ( 20 °C)
- Melting Point:30 °C
- Refractive Index:~1.358
- Boiling Point:100 °C at 760 mmHg
- PSA:18.46000
- Density:1.71 g/mL at 25 °C
- LogP:1.25040
Calcium chloride dihydrate(Cas 10035-04-8) Usage
|
Reference |
https://en.wikipedia.org/wiki/Calcium_chloride https://pubchem.ncbi.nlm.nih.gov/compound/Calcium_dichloride#section=Information-Sources https://www.drugs.com/pro/calcium-chloride.html |
|
Definition |
ChEBI: A hydrate that is the dihydrate form of calcium chloride. |
|
Brand name |
Cal Plus (Mallinckrodt). |
|
Biological Functions |
Calcium chloride is a commonly used reagent in biochemistry. Calcium plays important roles in many biological processes, including signal transduction, muscle contraction, and maintenance of cell membrane and cell wall stability.Extensive reviews of the experimental measurement of biological calcium have been published. Calcium chloride is used in the preparation and transformation of competent E. coli and in the transfection of eukaryotic cells with either plasmid DNA or high molecular weight genomic DNA.The CaCl2-mediated electroporation of E. coli with the plasmid DNA pBR322 has been studied.A protocol for the concentration of virus vectors that uses CaCl2 has been published. CaCl2 has been used in the stabilization and twodimensional crystallization of the NADH-ubiquinone oxidoreductase (complex I) from Escherichia coli. The crystallization of porcine pancreatic elastase in the presence of CaCl2 in the presence of sodium citrate reveals binding of calcium in the metal binding site of the protein.The effect of CaCl2 on the total fluorescence in the polymerication of the tubulin-like FtsZ division protein of Escherichia coli has been studied by a multiwell fluorescent assay. |
|
General Description |
ACS Reagent Grade. |
|
Biochem/physiol Actions |
Calcium chloride act as precipitants during protein crystallization process. It also enhances the competence of Escherichia coli cells as well as strengthened β-lactoglobulin gels by forming crossbridges. |
|
Potential Exposure |
Calcium chloride is used as road salt for melting snow, a drying agent in desiccators, for dehydrating organic liquids and gases, in refrigeration brines and antifreeze, as a dust-proofing agent, food additives, concrete hardening accelerator, and others. May react with strong oxidizers. |
|
Shipping |
There are no label or maximum shipping quantity requirements set by DOT. |
|
Purification Methods |
Crystallise it from ethanol. It is hygroscopic. It loses H2O at 200o so it can be dried at high temperatures to dehydrate it. The hexahydrate [7774-34-7] has m 30o and d 1.67. |
|
Properties and Applications |
ITEMS SPECIFICATION APPEARANCE WHITE,HARD ODORLESS FLAKE, POWDER,PELLET,GRANULE CALCIUM CHLORIDE(As CaCl2) 77% min MAGNESIUM&ALKALI METAL SALT (As NaCl) 4.0% max WATER INSOLUBLE MATTER 0.2% max ALKALINITY(As Ca(OH)2) 0.15% max SULFATE (As CaSO4) 0.25% max pH VALUE 7-11 As 5 ppm max Pb 10 ppm max Fe 10 ppm max |
|
ITEMS |
SPECIFICATION |
|
APPEARANCE |
WHITE,HARD ODORLESS FLAKE, POWDER,PELLET,GRANULE |
|
CALCIUM CHLORIDE(As CaCl2) |
77% min |
|
MAGNESIUM&ALKALI METAL SALT (As NaCl) |
4.0% max |
|
WATER INSOLUBLE MATTER |
0.2% max |
|
ALKALINITY(As Ca(OH)2) |
0.15% max |
|
SULFATE (As CaSO4) |
0.25% max |
|
pH VALUE |
7-11 |
|
As |
5 ppm max |
|
Pb |
10 ppm max |
|
Fe |
10 ppm max |
|
Incompatibilities |
The solution in water is a weak base. Reacts with zinc in presence of moisture, forming highlyflammable hydrogen gas. Dissolves violently in water with generation of much heat. Incompatible with water, bromine trifluoride; 2-furan, percarboxylic acid. May attack some building materials and metals in the presence of moisture. |
InChI:InChI:1S/Ca.2ClH.2H2O/h;2*1H;2*1H2/q+2;;;;/p-2
Relevant Products
-
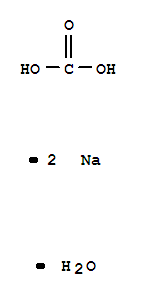
Sodium carbonate, monohydrate
CAS:5968-11-6
-
Antimony (III) oxide
CAS:1309-64-4
-
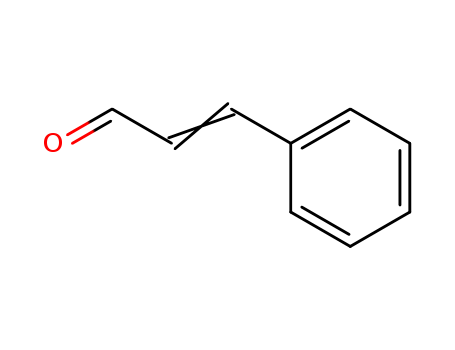
Cinnamaldehyde
CAS:104-55-2