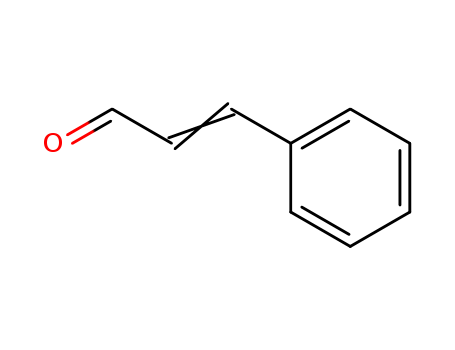
104-55-2
- Product Name:Cinnamaldehyde
- Molecular Formula:C9H8O
- Purity:99%
- Molecular Weight:132.162
Product Details;
CasNo: 104-55-2
Molecular Formula: C9H8O
Appearance: yellow liquid with an odour of cinnamon
Factory Sells Best Quality Cinnamaldehyde 104-55-2 with stock
- Molecular Formula:C9H8O
- Molecular Weight:132.162
- Appearance/Colour:yellow liquid with an odour of cinnamon
- Vapor Pressure:0.0265mmHg at 25°C
- Melting Point:-9 - -4 °C(lit.)
- Refractive Index:1.6210
- Boiling Point:246.8 °C at 760 mmHg
- PKA:0[at 20 ℃]
- Flash Point:71.1 °C
- PSA:17.07000
- Density:1.034 g/cm3
- LogP:1.89870
Cinnamaldehyde(Cas 104-55-2) Usage
|
Chemical Description |
Cinnamaldehyde and crotonaldehyde are specific examples of a,b-unsaturated aldehydes. |
|
Air & Water Reactions |
Thickens on exposure to air. May be unstable to prolonged exposure to air. Slightly water soluble . |
|
Reactivity Profile |
Cinnamaldehyde reacts with sodium hydroxide owing to aerobic oxidation. |
|
Health Hazard |
Cinnamaldehyde can cause moderate to severeskin irritation. Exposure to 40 mg in48 hours produced a severe irritation effecton human skin. The toxicity of this compoundwas low to moderate on test subjects,depending on the species and the toxicroutes. However, when given by oral routein large amounts, its poisoning effect wassevere. Amounts greater than 1500 mg/kghave produced a wide range of toxic effectsin rats, mice, and guinea pigs. The symptomswere respiratory stimulation, somnolence,convulsion, ataxia, coma, hypermotility, anddiarrhea.LD50 value, oral (guinea pigs): 1150 mg/kgCinnamaldehyde is a mutagen. Its carcinogeniceffect is not established. |
|
Fire Hazard |
Cinnamaldehyde is combustible. |
|
Trade name |
ADIOS?; ZIMTALDEHYDE?; ZIMTALDEHYDE? LIGHT |
|
Contact allergens |
This perfumed molecule is used as a fragrance in perfumes, a flavoring agent in soft drinks, ice creams, dentifrices, pastries, chewing-gum, etc. It can induce both contact urticaria and delayed-type reactions. It can be responsible for dermatitis in the perfume industry or in food handlers. Cinnamic aldehyde is contained in “fragrance mix.” As a fragrance allergen, it has to be mentioned by name in cosmetics within the EU. |
|
Anticancer Research |
This is promising in antitumor activity against NSCLC cells. The cells were inducedin apoptosis and also the epithelial-mesenchymal transition was reversed affectingthe Wnt/b-catenin pathway (Bouyahya et al. 2016). |
|
Safety Profile |
Poison by intravenous and parenteral routes. Moderately toxic by ingestion and intraperitoneal routes. A severe human skin irritant. Mutation data reported. Combustible liquid. May ipte after a delay period in contact with NaOH. When heated to decomposition it emits acrid smoke and fumes. See also ALDEHYDES. |
|
Synthesis |
By isolation from natural sources; synthetically, by condensation of benzaldehyde with acetaldehyde in the presence of sodium or calcium hydroxide. |
|
Potential Exposure |
Botanical fungicide and insecticide. Used as an antifungal agent, corn rootworm attractant, and dog and cat repellent. Can be used on soil casing for mushrooms, row crops, turf, and all food commodities. Not listed for use in EU countries. |
|
Shipping |
UN1989 Aldehydes, n.o.s., Hazard Class: 3; Labels: 3-Flammable liquid |
|
Incompatibilities |
Aldehydes are frequently involved in selfcondensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, ketones, azo dyes, caustics, boranes, hydrazines |
|
Waste Disposal |
Incineration. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. |
|
Aroma threshold values |
Detection at 50 to 750 ppb. |
|
Taste threshold values |
Taste characteristics at 0.5 ppm: spicy, cinnamon and cinnamon bark. |
|
General Description |
Yellow oily liquid with a cinnamon odor and sweet taste. |
|
Agricultural Uses |
Fungicide, Insecticide: Used as an antifungal agent, corn rootworm attractant, and dog and cat repellent. Can be used on soil casing for mushrooms, row crops, turf and all food commodities. Not listed for use in EU countries. |
InChI:InChI=1/C9H8O/c10-8-4-7-9-5-2-1-3-6-9/h1-8H/b7-4-
104-55-2 Relevant articles
Chemoselective deprotection of 1,3-oxathiolanes using Amberlyst 15 and glyoxylic acid under solvent free conditions
Chavan,Soni,Kamat
, p. 1251 - 1252 (2001)
Carbonyl compounds were regenerated from...
Organoselenium-Catalyzed Oxidative Allylic Fluorination with Electrophilic N-F Reagent
Guo, Ruizhi,Huang, Jiachen,Zhao, Xiaodan
, p. 926 - 930 (2018)
An efficient route of organoselenium-cat...
Highly enantioselective control of dynamic cascade transformations by dual catalysis: Asymmetric synthesis of polysubstituted spirocyclic oxindoles
Afewerki, Samson,Ma, Guangning,Ibrahem, Ismail,Liu, Leifeng,Sun, Junliang,Crdova, Armando
, p. 1266 - 1272 (2015)
The highly enantioselective (up to >99.5...
Retro-Diels-Alder Cleavage of endo-Bicyclohepta-5-en-2-ol
Prasad, J. V. N. Vara,Iyer, Padmini,Pillai, C. N.
, p. 1380 - 1381 (1982)
endo-Bicyclohepta-5-en-2-ol (1a) on reac...
A convenient oxidative deprotection of tetrahydropyranyl ethers with iron (III) nitrate and clay under microwave irradiation in solvent free conditions
Heravi, Majid M.,Ajami, Dariush,Mojtahedi, Mohammad M.,Ghassemzadeh, Mitra
, p. 561 - 562 (1999)
The efficient and environmentally benign...
Aspects of Selective Oxidation and Ammoxidation Mechanisms over Bismuth Molybdate Catalysts. 3. Substituent Effects in the Selective Oxidation of Allylbenzenes
Burrington, J. D.,Kartisek, C. T.,Grasselli, R. K.
, p. 1877 - 1882 (1981)
The relative rates of catalytic oxidatio...
Silica gel supported chromium trioxide: An efficient reagent for oxidative cleavage of oximes to carbonyl compounds under mild condition
Bendale, Pravin M.,Khadilkar, Bhushan M.
, p. 665 - 669 (2000)
A facile, efficient oxidative deblocking...
Silver(i)-catalysed carboxylative cyclisation of primary propargylic amines in neat water using potassium bicarbonate as a carboxyl source: An environment-friendly synthesis of: Z -5-alkylidene-1,3-oxazolidin-2-ones
Qin, Jian-Feng,Wang, Bing,Lin, Guo-Qiang
, p. 4656 - 4661 (2019)
Herein, we report a mild and environment...
Preparation and characterization of laccases immobilized on magnetic nanoparticles and their application as a recyclable nanobiocatalyst for the aerobic oxidation of alcohols in the presence of TEMPO
Rouhani, Shamila,Rostami, Amin,Salimi, Abdollah
, p. 26709 - 26718 (2016)
Laccase from Trametes versicolor was imm...
Efficient catalytic systems based on cobalt for oxidation of ethylbenzene, cyclohexene and oximes in the presence of N-hydroxyphthalimide
Habibi,Faraji,Arshadi,Heydari,Gil
, p. 282 - 292 (2013)
The selective oxidation of ethylbenzene ...
Catalytic oxidation of aromatic oxygenates by the heterogeneous catalyst Co-ZIF-9
Zakzeski, Joseph,Dbczak, Agnieszka,Bruijnincx, Pieter C.A.,Weckhuysen, Bert M.
, p. 79 - 85 (2011)
The catalytic activity of Co-ZIF-9, a ze...
Studies on Transition-Metal Oxo and Nitrido Complexes. Part 11. New Oxo Complexes of Ruthenium as Aerobically assisted Oxidants, and the X-Ray Crystal Structure of *3.5H2O
Dengel, Andrew C.,El-Hendawy, Ahmed M.,Griffith, William P.,O'Mahoney, Caroline A.,Williams, David J.
, p. 737 - 742 (1990)
The new complexes t-py), nicotinic acid...
-
Lalancette et al.
, p. 3058 (1972)
-
Design, synthesis, molecular modelling, and in vitro evaluation of tricyclic coumarins against Trypanosoma cruzi
Coelho, Gleicekelly Silva,Andrade, Josimara Souza,Xavier, Viviane Flores,Sales Junior, Policarpo Ademar,Rodrigues de Araujo, Barbara Caroline,Fonseca, Kátia da Silva,Caetano, Melissa Soares,Murta, Silvane Maria Fonseca,Vieira, Paula Melo,Carneiro, Claudia Martins,Taylor, Jason Guy
, p. 337 - 350 (2019)
Chagas disease is caused by infection wi...
Controllable Encapsulation of "clean" Metal Clusters within MOFs through Kinetic Modulation: Towards Advanced Heterogeneous Nanocatalysts
Liu, Hongli,Chang, Lina,Bai, Cuihua,Chen, Liyu,Luque, Rafael,Li, Yingwei
, p. 5019 - 5023 (2016)
Surfactant-free tiny Pt clusters were su...
An enantioselective three-component reaction of diazoacetates with indoles and enals by iridium/iminium co-catalysis
Li, Mingfeng,Guo, Xin,Jin, Weifeng,Zheng, Qing,Liu, Shunying,Hu, Wenhao
, p. 2736 - 2739 (2016)
The first example of chiral secondary am...
Synthesis, characterization, and catalytic activity of a water soluble copper(II) and nickel(II) heterobimetallic complex [CuNi(μ-OH)(μ-OH2)(μ-OAc)(bpy)2](ClO4)2 in aqueous medium in the absence of a base and co-catalyst
Lal, Ram A.,Kumar, Arvind,Syiemlieh, Ibanphylla,Kurbah, Sunshine D.
, p. 2722 - 2735 (2017)
A copper(II)–nickel(II)-based catalyst s...
Utilisation of gold nanoparticles on amine-functionalised UiO-66 (NH2-UiO-66) nanocrystals for selective tandem catalytic reactions
Hinde, Christopher S.,Webb, William R.,Chew, Benny K. J.,Tan, Hui Ru,Zhang, Wen-Hua,Hor, T. S. Andy,Raja, Robert
, p. 6557 - 6560 (2016)
Colloidal deposition of gold nanoparticl...
Synthetic scope of Ru(OH)x/Al2O3-catalyzed hydrogen-transfer reactions: An application to reduction of allylic alcohols by a sequential process of isomerization/meerwein-ponndorf-verley-iype reduction
Kim, Jung Won,Koike, Takeshi,Kotani, Miyuki,Yamaguchi, Kazuya,Mizuno, Noritaka
, p. 4104 - 4109 (2008)
Reduction of allylic alcohols can be pro...
Au-Pd nanoparticles on layered double hydroxide: Highly active catalyst for aerobic oxidation of alcohols in aqueous phase
Shi, Yu,Yang, Hanmin,Zhao, Xiuge,Cao, Ting,Chen, Jizhong,Zhu, Wenwen,Yu, Yinyin,Hou, Zhenshan
, p. 142 - 146 (2012)
Au-Pd bimetal catalysts supported on lay...
Oxidation of alcohols to carbonyl compounds with a new potassium permanganate adsorbed on Kieselguhr reagent
Lou, Ji-Dong,Lou, Wen-Xing
, p. 3697 - 3699 (1997)
A new reagent, potassium permanganate ad...
-
Newman
, p. 2254 (1973)
-
Highly efficient, organocatalytic aerobic alcohol oxidation
Shibuya, Masatoshi,Osada, Yuji,Sasano, Yusuke,Tomizawa, Masaki,Iwabuchi, Yoshiharu
, p. 6497 - 6500 (2011)
5-Fluoro-2-azaadamantane N-oxyl (5-F-AZA...
A convenient oxidative demasking of 1,3-dithiolanes and dithianes to carbonyl compounds with TBHP
Barhate, Nivrutti B,Shinde, Popat D,Mahajan, Vishal A,Wakharkar, Radhika D
, p. 6031 - 6033 (2002)
Regeneration of carbonyl compounds from ...
Unveiling of a Trinuclear Cyclic Peroxidovanadate: A Potential Oxidant in Vanadium-Catalyzed Reactions
Krivosudsky, Luká?,Schwendt, Peter,Gyepes, Róbert
, p. 6306 - 6311 (2015)
The first peroxidovanadium trimer was pr...
-
Sadler,I.H.,Stewart,J.A.G.
, p. 773 - 774 (1969)
-
Development of manganese(VI) oxidising agents soluble in organic solvents
Ellis, Rhys,Lee, Kee-Han,Ainsworth, Matthew,Kerr, Alexander,Viseux, Eddy M. E.
, p. 1371 - 1373 (2012)
Two manganate(VI) reagents have been pre...
OXIDATION OF ALCOHOLS TO CARBONYL COMPOUNDS WITH CHROMIUM(V) REAGENTS
Chakraborty, T. K.,Chandrasekaran, S.
, p. 1583 - 1586 (1980)
Two pentavalent chromium complexes have ...
Barium dichromate [BaCr2O7], a mild reagent for oxidation of alcohols to their corresponding carbonyls in non-aqueous polar aprotic media
Mottaghinejad, Enayatollah,Shaafi,Ghasemzadeh
, p. 8823 - 8824 (2004)
Barium dichromate is used as a mild oxid...
Oxidation of alcohols to carbonyl compounds under solvent-free conditions using permanganate supported on alumina
Hajipour, Abdol Reza,Mallakpour, Shadpour E.,Imanzadeh, Gholamhasan
, p. 99 - 100 (1999)
A manupulatively simple and rapid method...
Selective aerobic oxidation of alcohols by using manganese oxide nanoparticles as an efficient heterogeneous catalyst
Sun, Hua-Yin,Hua, Qing,Guo, Feng-Feng,Wang, Zhi-Yong,Huang, Wei-Xin
, p. 569 - 573 (2012)
Manganese oxide (Mn3O4) nanoparticles ha...
An easy and fast method for conversion of oximes to the corresponding carbonyl compounds under microwave irradiation
Hajipour,Mallakpour,Khoee
, p. 9 - 15 (2002)
1-Benzyl-4-aza-1-azoniabicyclo[2.2.2]oct...
Immobilized magnetic nano catalyst for oxidation of alcohol
Bhat, Pooja B.,Rajarao, Ravindra,Sahajwalla, Veena,Bhat, Badekai Ramachandra
, p. 42 - 49 (2015)
Covalent attachment of Schiff base on ma...
BIS(p-METHOXYPHENYL)SELENOXIDE AS A COOXIDANT FOR SELENIUM DIOXIDE OXIDATION OF BENZYL ALCOHOLS
Ogura, Fumio,Otsubo, Tetsuo,Ariyoshi, Kimio,Yamaguchi, Hachiro
, p. 1833 - 1834 (1983)
The title selenoxide provides a new oxid...
Effective oxidation of alcohols under heterogeneous conditions with a new reagent: Manganese dioxide supported on graphite
Lou, Ji-Dong,Lu, Xiu Lian,Huang, Li-Hong,Wang, Qiang,Zou, Xiao-Nan
, p. 1342 - 1345 (2011)
A new reagent, manganese dioxide support...
Mild, green copper/4-dimethylaminopyridine catalysed aerobic oxidation of alcohols mediated by nitroxyl radicals in water
Zhang, Guoqi,Yang, Chengxiong,Liu,Li, Li,Golen, James A.,Rheingold, Arnold L.
, p. 61907 - 61911 (2014)
A novel copper/4-dimethylaminopyridine c...
Highly Enantioselective Synthesis of Alkylpyridine Derivatives through a Michael/Michael/Aldol Cascade Reaction
Meazza, Marta,Potter, Michael,Pitak, Mateusz B.,Coles, Simon J.,Mazzanti, Andrea,Rios, Ramon
, p. 719 - 725 (2017)
A method for the synthesis of pyridine d...
An Fe3O4@P4VP@FeCl3 core-shell heterogeneous catalyst for aerobic oxidation of alcohols and benzylic oxidation reaction
Li, Ruilian,Zhao, Jian,Yang, Fengxia,Zhang, Yingchao,Ramella, Daniele,Peng, Yu,Luan, Yi
, p. 51142 - 51150 (2017)
A novel magnetic Fe3O4@P4VP(poly(4-vinyl...
From surface-inspired oxovanadium silsesquioxane models to active catalysts for the oxidation of alcohols with O2-The cinnamic acid/ metavanadate system
Ohde, Christian,Limberg, Christian
, p. 6892 - 6899 (2010)
Silsesquioxane dioxovanadate(V) complexe...
-
Wayaku et al.
, p. 1957 (1975)
-
Palladium-Catalyzed Allylic C-H Oxidative Annulation for Assembly of Functionalized 2-Substituted Quinoline Derivatives
Li, Chunsheng,Li, Jianxiao,An, Yanni,Peng, Jianwen,Wu, Wanqing,Jiang, Huanfeng
, p. 12189 - 12196 (2016)
An efficient and practical palladium-cat...
A Copper(I) Complex with Two Unpaired Electrons, Synthesised by Oxidation of a Copper(II) Complex with Two Redox-Active Ligands
Asyuda, Andika,Enders, Markus,Himmel, Hans-J?rg,Kaifer, Elisabeth,Werr, Marco,Zharnikov, Michael
, p. 23451 - 23462 (2021)
Two homoleptic copper(II) complexes [Cu(...
Direct synthesis of Fe(III) immobilized Zr-based metal–organic framework for aerobic oxidation reaction
Shu, Xin,Yu, Ying,Jiang, Yi,Luan, Yi,Ramella, Daniele
, (2017)
A Zr-based metal–organic framework with ...
-
Ficini et al.
, p. 3589 (1977)
-
The Aldol Reaction under High-Intensity Ultrasound: A Novel Approach to an Old Reaction
Cravotto, Giancarlo,Demetri, Alberto,Nano, Gian Mario,Palmisano, Giovanni,Penoni, Andrea,Tagliapietra, Silvia
, p. 4438 - 4444 (2003)
We have employed high-intensity ultrasou...
Evolution of physical and photocatalytic properties of new Zn(II) and Ru(II) complexes
Gugulothu, Venkanna,Ahemed, Jakeer,Subburu, Mahesh,Yadagiri, Bhongiri,Mittal, Ritu,Prabhakar, Chetti,Pola, Someshwar
, p. 412 - 423 (2019)
Synthesis of Zn(II) and Ru(II) complexes...
Organische Synthesen mit Uebergangsmetall-Komplexen. LXV. Aldehyde durch Hydrolyse der M=C-Bindung von Alkoxycarben-Chromkomplexen mit Wasser / Urotropin. Ein zweikerniger verbrueckter (β-Amino)vinylcarben-Chromkomplex durch Fragmentierung von Urotropin
Aumann, Rudolf,Hinterding, Peter,Krueger, Carl,Goddard, Richard
, p. 145 - 150 (1993)
The Cr=C bonds of aryl- and alkenyl(etho...
Microwave-assisted oxidation of alcohols using wet alumina supported ammonium chlorochromate in solventless system
Heravi, Majid M.,Aghayan, Maryam M.
, p. 815 - 817 (1999)
A simple and selective method for the ox...
Shaken not stirred; Oxidation of alcohols with sodium dichromate
Lou, Ji-Dong,Gao, Chun-Ling,Ma, Yi-Chun,Huang, Li-Hong,Li, Li
, p. 311 - 313 (2006)
Efficient and selective oxidation of pri...
Aerobic alcohol oxidation catalyzed by polyester-based Pd(II) macrocomplexes
Giachi, Guido,Frediani, Marco,Oberhauser, Werner,Passaglia, Elisa
, p. 2725 - 2731 (2012)
A 1:2 mixture of the poly (l-lactide)-ba...
An efficient Pd@Pro-GO heterogeneous catalyst for the α, β-dehydrogenation of saturated aldehyde and ketones
Pan, Gao-Fei,Wang, Zhe,Chang, Yi-Yuan,Hao, Yue,Wang, Yi-Chen,Xing, Rui-Guang
supporting information, (2021/12/30)
An Efficient Pd@Pro-GO heterogeneous cat...
Radical induced disproportionation of alcohols assisted by iodide under acidic conditions
Huang, Yang,Jiang, Haiwei,Li, Teng,Peng, Yang,Rong, Nianxin,Shi, Hexian,Yang, Weiran
supporting information, p. 8108 - 8115 (2021/10/29)
The disproportionation of alcohols witho...
Nitrosoarene-Catalyzed HFIP-Assisted Transformation of Arylmethyl Halides to Aromatic Carbonyls under Aerobic Conditions
Pradhan, Suman,Sharma, Vishali,Chatterjee, Indranil
supporting information, p. 6148 - 6152 (2021/08/03)
A rare metal-free nucleophilic nitrosoar...
Method for preparing olefine aldehyde through catalytic oxidation of enol ether
-
Paragraph 0082-0088, (2021/06/23)
The invention relates to the technical f...
104-55-2 Process route
-
-
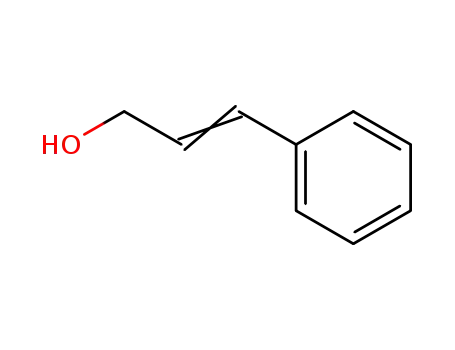
104-54-1
3-Phenylpropenol

-
-
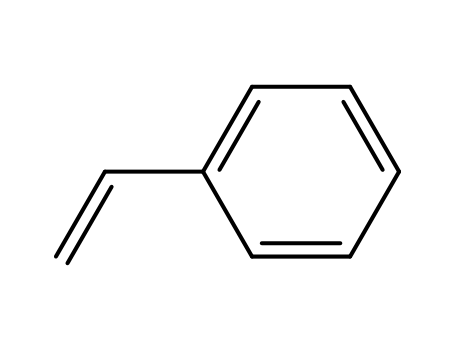
100-42-5,25038-60-2,25247-68-1,28213-80-1,28325-75-9,79637-11-9,9003-53-6
styrene

-
-
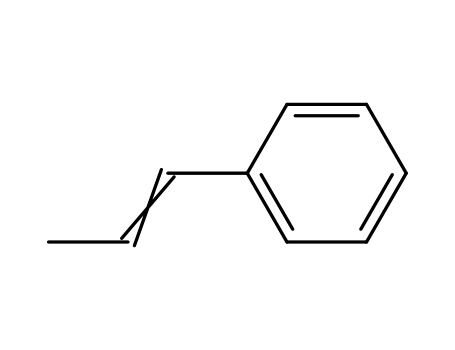
637-50-3
1-phenylpropene

-
-
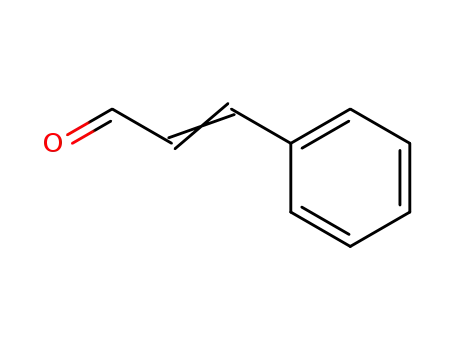
104-55-2
3-phenyl-propenal
| Conditions | Yield |
|---|---|
|
With
bis(1,5-cyclooctadiene)nickel (0); triphenylphosphine;
In
tetrahydrofuran;
at 30 ℃;
for 48h;
Product distribution;
|
53% 9% |
-
-
1295-35-8
bis(1,5-cyclooctadiene)nickel (0)

-

-
104-54-1
3-Phenylpropenol

-

-
100-42-5,25038-60-2,25247-68-1,28213-80-1,28325-75-9,79637-11-9,9003-53-6
styrene

-

-
637-50-3
1-phenylpropene

-
-
63576-88-5
Ni(C6H5CHCHCHO)(P(C6H5)3)2

-
-
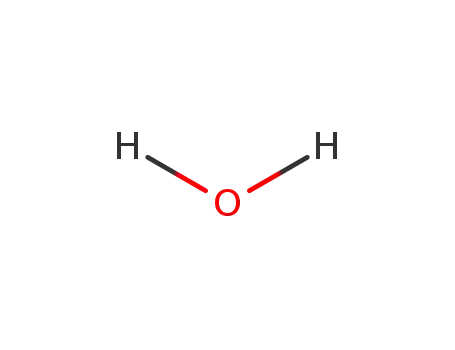
7732-18-5
water

-

-
104-55-2
3-phenyl-propenal
| Conditions | Yield |
|---|---|
|
With
triphenylphosphine;
In
tetrahydrofuran;
under N2 or argon or under vac., soln. of org. compd. (3.8 mol) added to mixt. of Ni(cod)2 and PPh3 (1.0/2.2 mol ratio), stirred at 30°Cfor 2 d; detn. by IR and NMR;
|
30% 53% 9% <1 51% |
104-55-2 Upstream products
-
91-22-5
quinoline
-
99-65-0
meta-dinitrobenzene
-
497-19-8
sodium carbonate
-
4407-36-7
(2E)-3-phenyl-2-propen-1-ol
104-55-2 Downstream products
-
66684-75-1
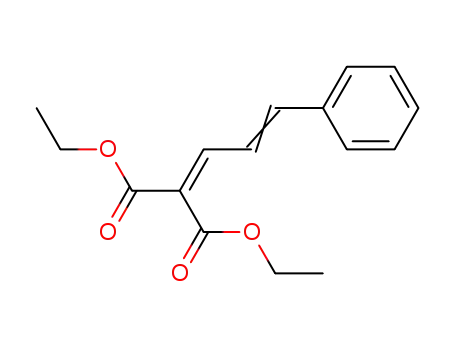
diethyl 2-[3-phenylprop-2-en-1-ylidene]propanedioate
-
22915-20-4
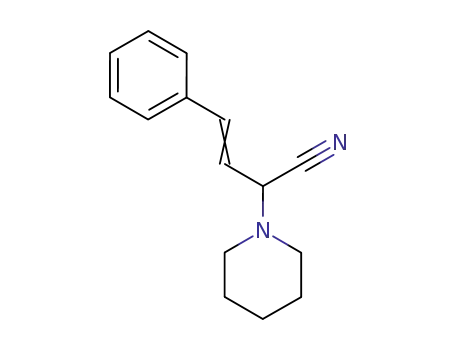
4-phenyl-2-piperidin-1-yl-but-3-enenitrile
-
676551-01-2
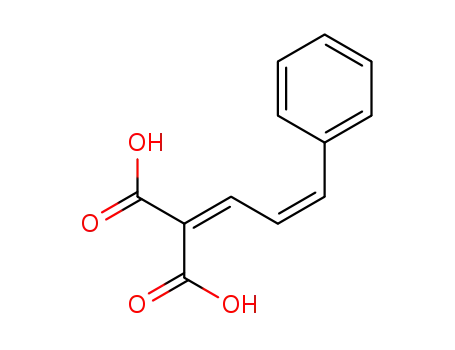
2-((Z)-3-Phenyl-allylidene)-malonic acid
-
52746-24-4
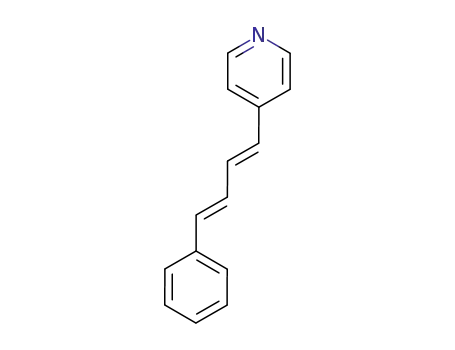
(E,E)-1-(4-pyridyl)-4-(phenyl)buta-1,3-diene
Relevant Products
-
Tesamorelin
CAS:218949-48-5
-
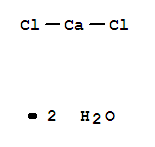
Calcium chloride dihydrate
CAS:10035-04-8
-
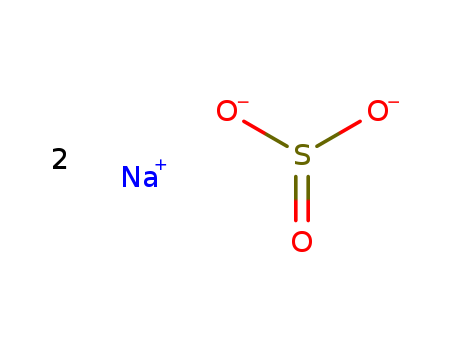
Sodium sulfite
CAS:7757-83-7