7487-88-9
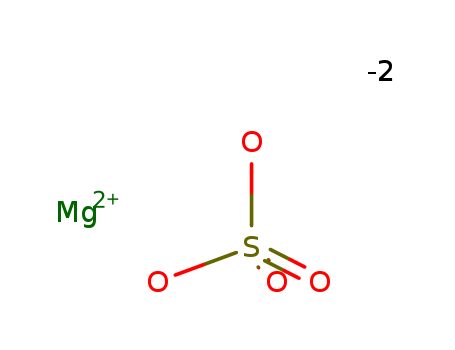
- Product Name:Magnesium sulfate
- Molecular Formula:MgSO4
- Purity:99%
- Molecular Weight:120.369
Product Details;
CasNo: 7487-88-9
Molecular Formula: MgSO4
Appearance: White crystalline powder
Manufacturer supply high quality Magnesium sulfate 7487-88-9 with GMP standards
- Molecular Formula:MgSO4
- Molecular Weight:120.369
- Appearance/Colour:White crystalline powder
- Melting Point:1124 °C
- Boiling Point:330 °C at 760 mmHg
- PSA:88.64000
- Density:2.66 g/cm3
- LogP:-0.25720
Magnesium sulfate(Cas 7487-88-9) Usage
|
Chemical Properties and Role |
Magnesium sulfate is a magnesium salt with sulfate as the counterion. It serves various roles such as an anticonvulsant, cardiovascular drug, calcium channel blocker, anesthetic, tocolytic agent, anti-arrhythmia drug, analgesic, and fertilizer. Magnesium plays a crucial role in the physiological function of the human body. |
|
Application in Regional Anesthesia |
Numerous studies have demonstrated the safety and effectiveness of adding magnesium sulfate to various regional anesthesia techniques. Meta-analyses have shown that combining magnesium sulfate with local anesthetics in nerve blocks can lead to prolonged postoperative analgesia. The addition of magnesium sulfate to bupivacaine during transversus abdominis plane (TAP) block and subclavian brachial plexus nerve block resulted in a longer duration of analgesia and reduced postoperative morphine requirements. |
|
Enhancement of Local Anesthetics in Nerve Blocks |
With the widespread use of ultrasound, nerve blocks have become more prevalent but are limited by shorter duration and suboptimal analgesia. Magnesium sulfate, as a local anesthetic adjuvant for peripheral nerve blocks, enhances the effects of local anesthetics. |
|
Neuroprotection in Preterm Birth |
Magnesium sulfate (MgSO4) has been proposed as a crucial intervention for preventing neurologic disability associated with preterm birth. It is readily accessible, cost-effective, and has been recommended as a mandatory component of managing inevitable preterm birth. MgSO4 administration has been shown to reduce the risk of severe neurologic deficits, particularly cerebral palsy, in appropriately selected patients. Guidelines recommend a standard regimen of 4g intravenous loading dose followed by 1g/h maintenance dose for up to 24 hours, but recent studies suggest higher doses may provide maximum protective effect. |
|
General Description |
Magnesium sulfate, also known as Epsom salt, is a chemical compound made up of magnesium, sulfur, and oxygen. It is commonly used in medicine as a magnesium supplement and as a treatment for magnesium deficiency. It is also used as a laxative and as a remedy for constipation. In addition to its medical uses, magnesium sulfate is used in agriculture as a fertilizer, in food as a flavor enhancer, and in various industrial applications. It is also used in the production of some types of hard cheese and tofu, and as a coagulant in the manufacture of tofu. |
InChI:InChI=1/Mg.H2O4S.2H/c;1-5(2,3)4;;/h;(H2,1,2,3,4);;/p-2/rH2Mg.H2O4S/c;1-5(2,3)4/h1H2;(H2,1,2,3,4)/p-2
7487-88-9 Relevant articles
Kinetics study of the SO2 sorption by Brazilian dolomite using thermogravimetry
Crnkovic,Milioli,Pagliuso
, p. 161 - 166 (2006)
Sulfur emission in coal power generation...
Molten potassium pyrosulphate: Reactions of oxides of ten main-group elements
Salem,Tariq
, p. 123 - 125 (1997)
The reactions of MgO, CaO, SrO, BaO, ZnO...
Differential thermal study of the interactions between sulphates, oxides and ferrites
Boyanov
, p. 109 - 115 (1997)
The solid state interactions in the ZnSO...
Solvent-assisted construction of diverse Mg-TDC coordination polymers
Song, Ying,Feng, Mei-Ling,Wu, Zhao-Feng,Huang, Xiao-Ying
, p. 1348 - 1357 (2015)
Upon alteration of selected solvents, th...
Temperature dependence of electric permittivity of linear dielectrics with ionic and polar covalent bonds
Napijalo,Nikolic,Dojcilovic,Napijalo,Novakovic
, p. 1255 - 1258 (1998)
Results are presented of experimental ve...
A novel method of non-violent dissolution of sodium metal in a concentrated aqueous solution of Epsom salt
Lakshmanan,Prasad,Ponraju,Krishnan
, p. 3460 - 3468 (2004)
A new technique of non-violent and fast ...
Kinetic model for the reaction of ilmenite with sulphuric acid
Jablonski,Przepiera
, p. 583 - 590 (2001)
The kinetic of the reaction ilmenite wit...
THREE-DIMENSIONAL CROSSLINKER COMPOSITION AND METHOD OF MANUFACTURING ELECTRONIC DEVICES USING THE SAME
-
, (2022/01/04)
The inventive concept relates to a three...
Kinetics of the topochemical reaction of the solid solutions of magnesia spinels: Mg(Cr0.5Fe0.5)2O4, Mg(Al0.5Cr0.5)2O4, Mg(Al0.5Fe0.5)2O4 with sulphur oxides
Gerle, Anna,Piotrowski, Jerzy,Podwórny, Jacek
, (2019/08/01)
Spinel-containing materials belong to an...
COMPOUND AND ORGANIC LIGHT-EMITTING DEVICE INCLUDING THE SAME
-
, (2016/06/28)
The present disclosure relates to a comp...
7487-88-9 Process route
-
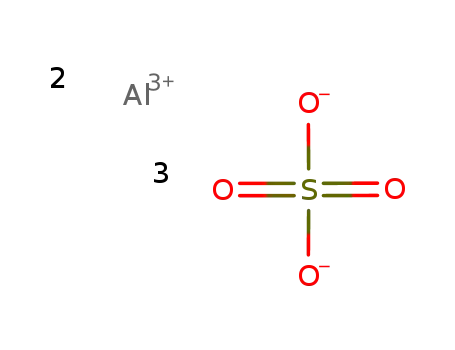
-
aluminum(III) sulfate

-
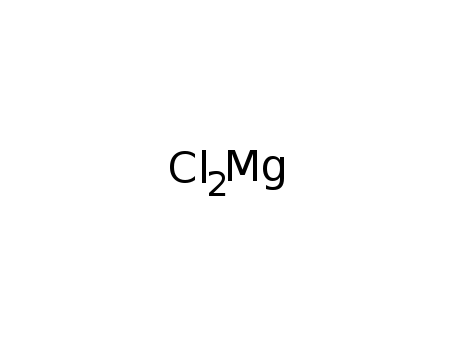
-
7786-30-3
magnesium chloride

-
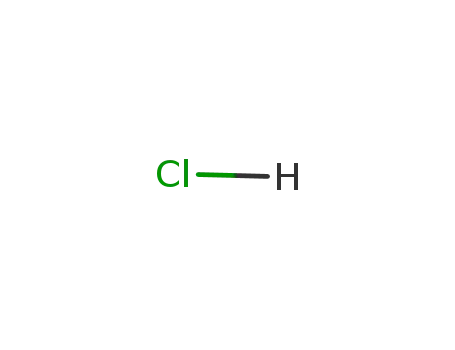
-
7647-01-0,15364-23-5
hydrogenchloride

-
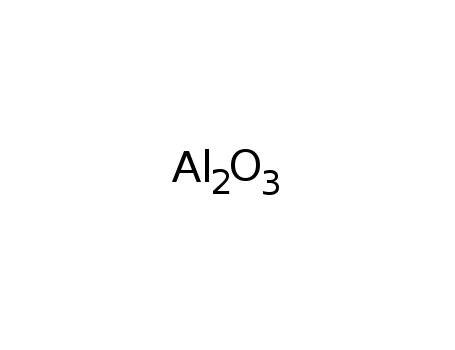
-
1333-84-2,1344-28-1
aluminum oxide

-
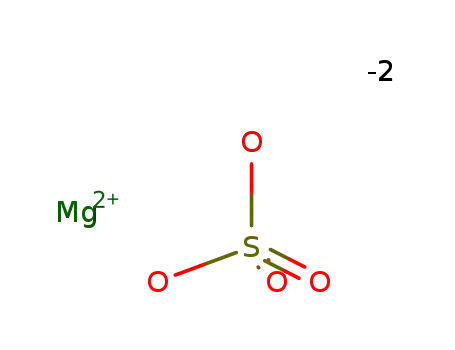
-
7487-88-9
magnesium sulfate
| Conditions | Yield |
|---|---|
|
|
|
|
In
neat (no solvent);
evapn. and heating;;
|
-
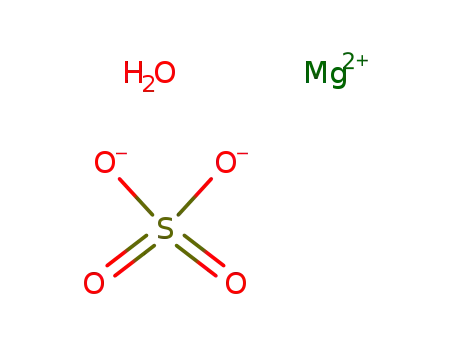
-
639819-42-4,13841-11-7
magnesium sulfate monohydrate

-
-
Mg2(OH)3I

-
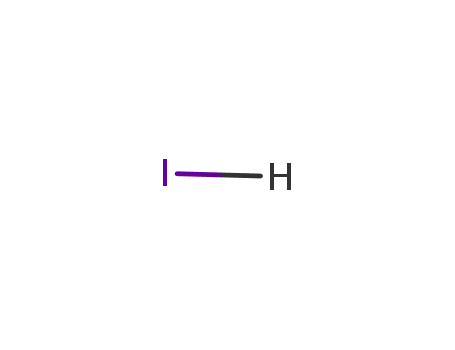
-
10034-85-2
hydrogen iodide

-
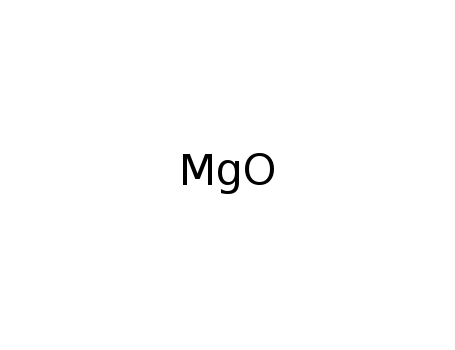
-
magnesium oxide

-

-
7487-88-9
magnesium sulfate
| Conditions | Yield |
|---|---|
|
byproducts: H2O; heated in N2 atm. at 350°C;
|
7487-88-9 Upstream products
-
7446-09-5
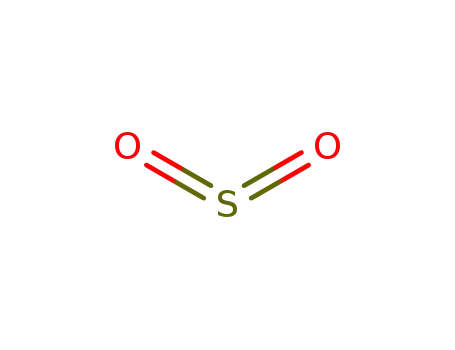
sulfur dioxide
-
80937-33-3
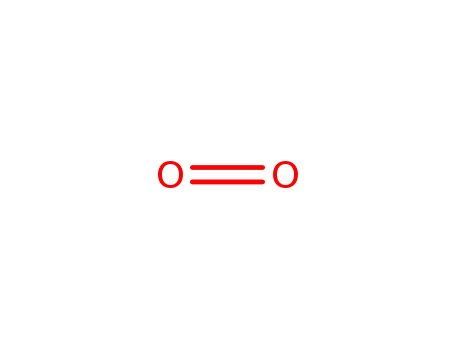
oxygen
-
7664-93-9
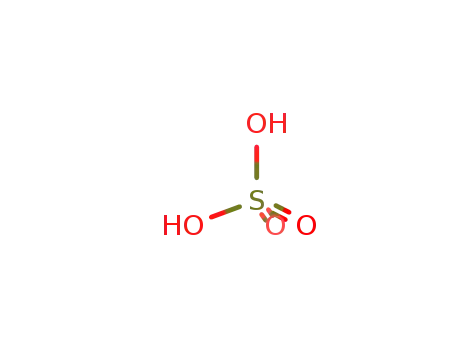
sulfuric acid
-
7681-38-1
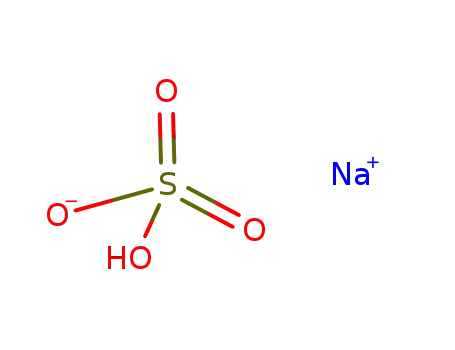
sodium hydrogen sulfate
7487-88-9 Downstream products
-
861321-52-0
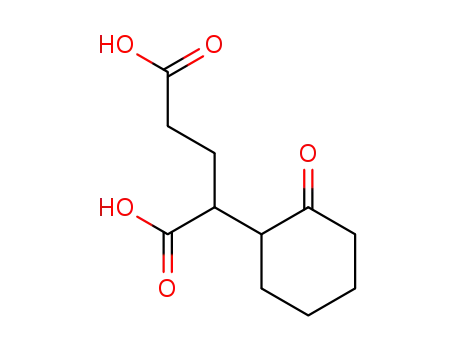
2-(2-oxo-cyclohexyl)-glutaric acid
-
18978-24-0
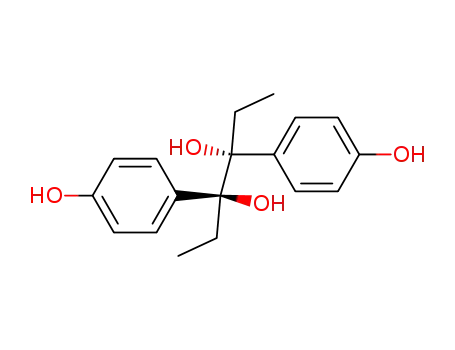
meso-3,4-bis-(4-hydroxy-phenyl)-hexane-3,4-diol
-
7507-01-9
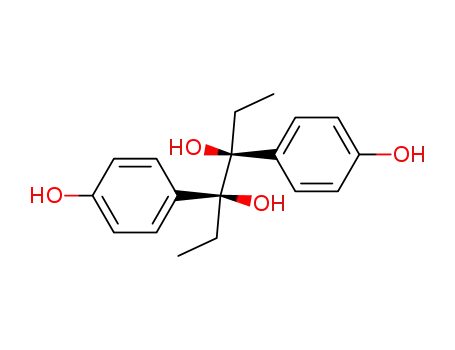
racem.-3.4-bis-(4-hydroxy-phenyl)-hexanediol-(3.4)
-
100-65-2
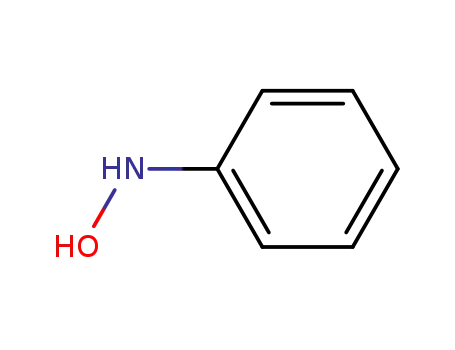
N-Phenylhydroxylamine
Relevant Products
-
PEG MGF
CAS:12020-86-9
-
D-Glucosamine hydrochloride
CAS:66-84-2
-
Atorvastatin calcium
CAS:134523-03-8