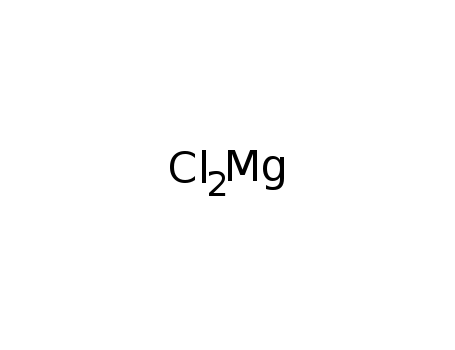
7786-30-3
- Product Name:Magnesium chloride
- Molecular Formula:Cl2Mg
- Purity:99%
- Molecular Weight:95.211
Product Details;
CasNo: 7786-30-3
Molecular Formula: Cl2Mg
Appearance: white powder
Chinese factory supply Magnesium chloride 7786-30-3 in stock with high standard
- Molecular Formula:Cl2Mg
- Molecular Weight:95.211
- Appearance/Colour:white powder
- Melting Point:714 °C(lit.)
- Refractive Index:n20/D 1.336
- Boiling Point:1412 °C
- PSA:0.00000
- Density:2.32 g/mL at 25 °C(lit.)
- LogP:-5.99200
Magnesium chloride(Cas 7786-30-3) Usage
|
General Description |
Magnesium chloride,MgCI2,also known as chloromagnesite, is a colorless crystalline solid. It is soluble in water and alcohol and is used in the ceramic and textile industries. Magnesium chloride is formed by heating hydrated magnesium chloride crystals in a current of dry hydrogen chloride or by heating magnesium ammonium chloride. Hydrated magnesium chloride, MgCI2·6H20 , also known as bischophite,is a white deliquescent solid formed by the reaction of magnesium carbonate(or hydroxide,oxide or metal)and hydrogen chloride. It is used in disinfectants, fire extinguishers, and paper manufacture. magnesium chloride flakes |
|
Definition |
magnesium chloride: A white solid compound, MgCl2. The anhydrous salt (hexagonal; r.d. 2.32; m.p.714°C; b.p. 1412°C) can be prepared by the direct combination of dry chlorine with magnesium: Mg(s) + Cl2(g) → MgCl2(s) The compound also occurs naturally as a constituent of carnallite(KCl.MgCl2). It is a deliquescent compound that commonly forms the hexahydrate, MgCl2.6H2O (monoclinic;r.d. 1.57). When heated, this hydrolyses to give magnesium oxide and hydrogen chloride gas. The fused chloride is electrolysed to produce magnesium and it is also used for fireproofing wood, in magnesia cements and artificial leather, and as a laxative. |
|
Preparation |
In the “Dow Process” (which is the electrolytic method of extracting bromine from brine), magnesium chloride is regenerated from magnesium hydroxide by hydrochloric acid: Mg(OH)2(s)+2HCl→MgCl2(aq)+2H2O It can also be prepared from MgCO3 by a similar reaction. Magnesium chloride is ionic and so will undergo electrolysis when it is molten. Electricity is carried by the movement of the ions within the melt and their discharge at the electrodes. However, solid magnesium chloride is a nonconductor of electricity because the ions are immobile. |
|
Hazard |
Toxic by ingestion. |
|
Flammability and Explosibility |
Nonflammable |
InChI:InChI=1/2ClH.Mg/h2*1H;/q;;+2/p-2
7786-30-3 Relevant articles
Controlled synthesis of Mg(OH)2 nanorods using basic magnesium chloride as precursor
Chai, Shu-Jing,Luo, Bi-Jun,Wu, Hai-Hong,Wu, Dan,Lu, Shao-Yan,Zhang, Qi
, p. 90 - 101 (2021/07/07)
Mg(OH)2 nanorods were successfully prepa...
NOVEL ORGANO-MAGNESIUM COMPOUNDS AND THEIR USE
-
Page/Page column 11; 15, (2021/11/26)
The present invention relates to novel o...
In-situ observation on the magnesiothermic reduction of TiCl4 around 800 °C by microfocus X-ray fluoroscopy
Kishimoto, Akihiro,Uda, Tetsuya
, (2021/05/06)
In the industrial smelting process for t...
Kinetically Controlled Low-Temperature Solid-State Metathesis of Manganese Nitride Mn3N2
Rognerud, Erik G.,Rom, Christopher L.,Todd, Paul K.,Singstock, Nicholas Ryan,Bartel, Christopher J.,Holder, Aaron M.,Neilson, James R.
, p. 7248 - 7254 (2019/09/30)
The synthesis of inorganic metal nitride...
7786-30-3 Process route
-
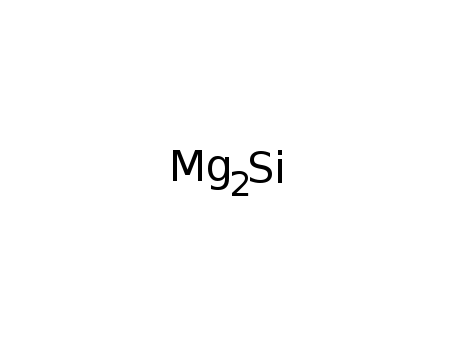
-
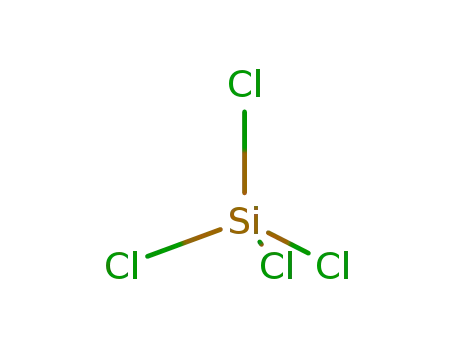
22831-39-6
magnesium silicide

-
-
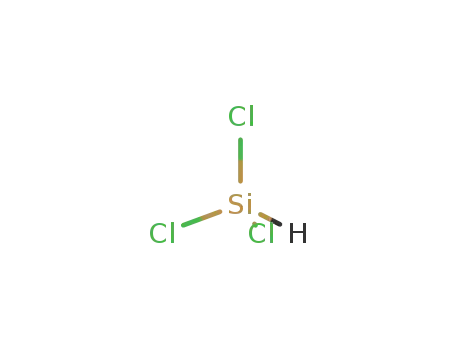
10025-78-2
trichlorosilane

-
-
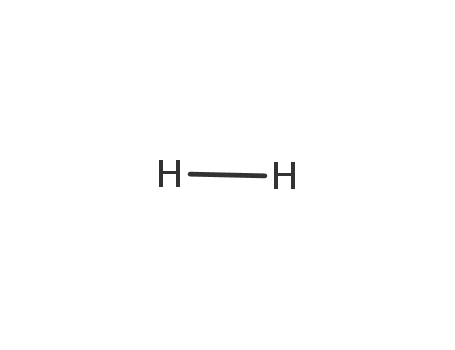
1333-74-0
hydrogen

-
-
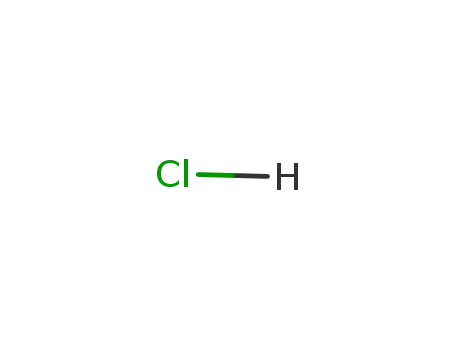
7647-01-0,15364-23-5
hydrogenchloride

-
-
10026-04-7,53609-55-5
tetrachlorosilane

-

-
7440-21-3
monosilane

-
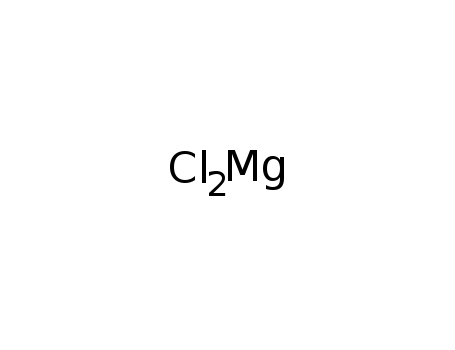
-
7786-30-3
magnesium chloride

-

-
7440-21-3
silicon
| Conditions | Yield |
|---|---|
|
In
gas;
heating SiHCl3 and H2 at 500°C in a reactor, passing over a Mg2Si charge which was heated upto 220°C, react. time: 4 h, condensing exit gases in liq. N2 traps; detn. by gas chromy., cooling the Mg2Si charge to room temp. under H2, deposition of Si on the reactor walls;
|
-
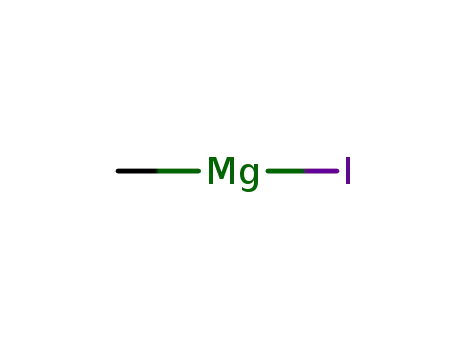
-
917-64-6
methyl magnesium iodide

-
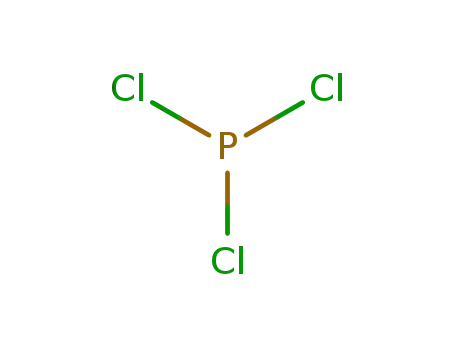
-
7719-12-2,52843-90-0
phosphorus trichloride

-
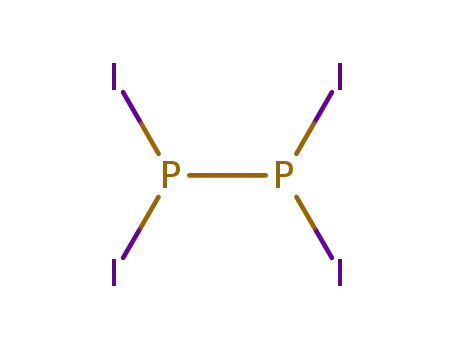
-
13455-00-0
diphosphorus tetraiodide

-
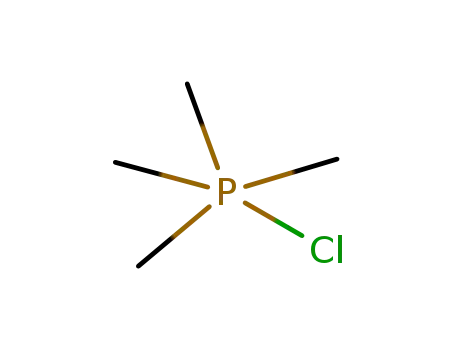
-
880343-42-0
chloro-tetramethyl-phosphorane

-
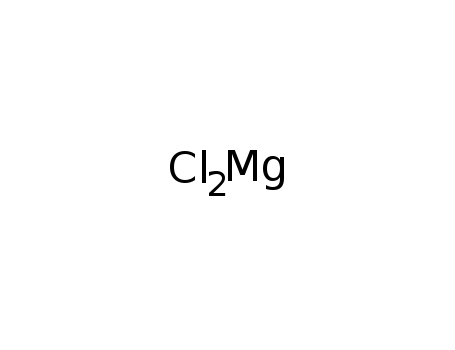
-
7786-30-3
magnesium chloride
| Conditions | Yield |
|---|---|
|
In
diethyl ether;
moderate reaction in ethereal soln. at -20°C, vigorous reaction at ambient temperature;;
|
|
|
In
diethyl ether;
moderate reaction in ethereal soln. at -20°C, vigorous reaction at ambient temperature;;
|
7786-30-3 Upstream products
-
2386-64-3
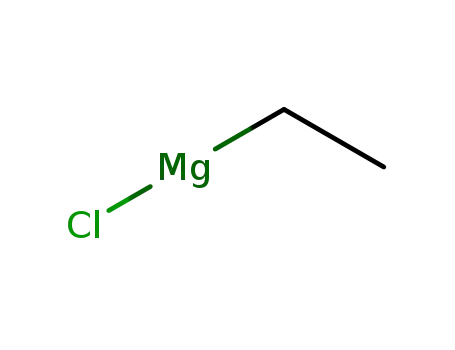
ethylmagnesium chloride
-
993-10-2
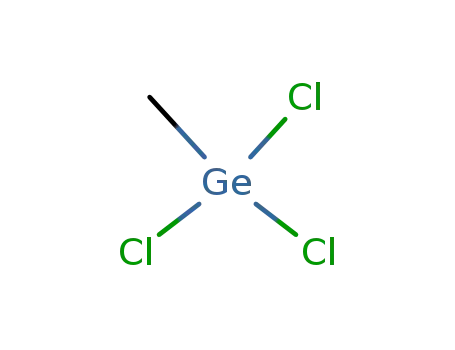
methyltrichlorogermane
-
1068-55-9
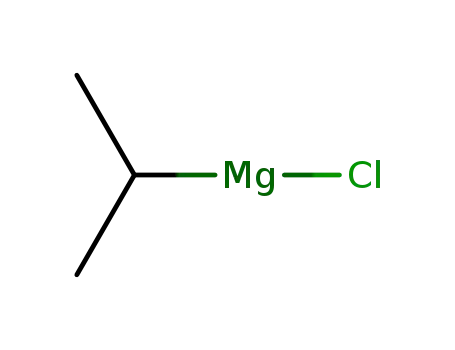
isopropylmagnesium chloride
-
97-93-8
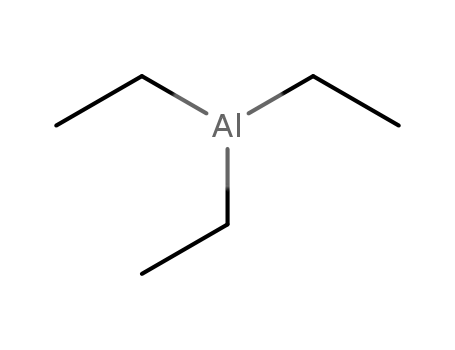
triethylaluminum
7786-30-3 Downstream products
-
557-27-7
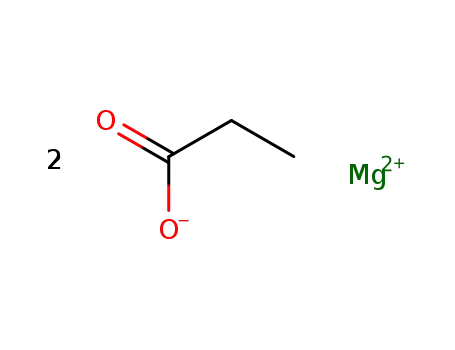
magnesium dipropionate
-
557-39-1
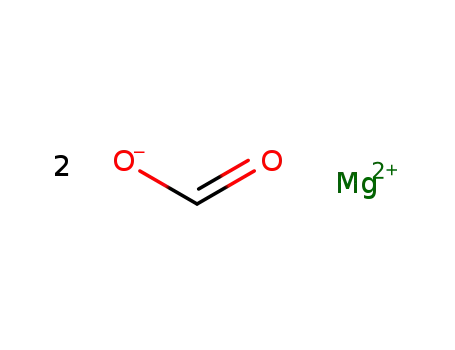
magnesium formate
-
10476-85-4
strontium chloride
-
7446-70-0
aluminium trichloride
Relevant Products
-
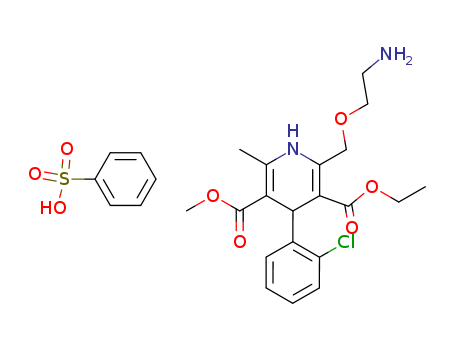
Amlodipine besylate
CAS:111470-99-6
-
Rosin
CAS:8050-09-7
-
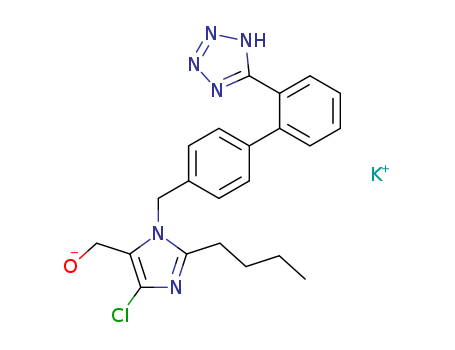
Losartan potassium
CAS:124750-99-8