6066-82-6
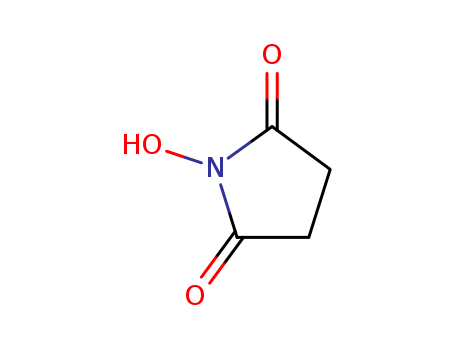
- Product Name:N-Hydroxysuccinimide
- Molecular Formula:C4H5NO3
- Purity:99%
- Molecular Weight:115.089
Product Details;
CasNo: 6066-82-6
Molecular Formula: C4H5NO3
Appearance: white to pale yellow
Reliable factory customized supply N-Hydroxysuccinimide 6066-82-6
- Molecular Formula:C4H5NO3
- Molecular Weight:115.089
- Appearance/Colour:white to pale yellow
- Vapor Pressure:0.00155mmHg at 25°C
- Melting Point:95-98 °C(lit.)
- Refractive Index:1.599
- Boiling Point:262.4 °C at 760 mmHg
- PKA:7.81±0.20(Predicted)
- Flash Point:112.5 °C
- PSA:57.61000
- Density:1.649 g/cm3
- LogP:-0.53750
N-Hydroxysuccinimide(Cas 6066-82-6) Usage
|
Chemical Description |
N-hydroxysuccinimide is a coupling reagent used in peptide synthesis. |
|
Synthesis |
N-Hydroxysuccinimide can be synthesized by heating succinic anhydride with hydroxylamine or hydroxylamine hydrochloride. |
|
Purification Methods |
Recrystallise the imide from EtOH/ethyl acetate [Manesis & Goodmen J Org Chem 52 5331 1987]. [Beilstein 21/9 V 498.] |
|
Application |
N-Hydroxysuccinimide has been used in the synthesis of intermediates such as:N-succinimidyl 3-(di-tert-butylfluorosilyl)benzoate4-[2,2-bis[(p-tolylsulfonyl)-methyl] acetyl]benzoic acid-NHS esterN-succinimidyl 3-iodobenzoateIt has also been used in a protocol for the surface modification of microchannels of a microfluidic-integrated surface plasmon resonance (SPR) platform for the detection and quantification of bacterial pathogens.Additive used in the carbodiimide method for improved amidations and peptide couplings.N-Hydroxysuccinimide can be used in the synthesis of N-hydroxysuccinimide ester via dehydration reaction in the presence of dicyclohexylcarbodiimide in carboxylic acid. |
InChI:InChI=1/C4H5NO3/c6-3-1-2-4(7)5(3)8/h8H,1-2H2
6066-82-6 Relevant articles
TRPA1 is activated by direct addition of cysteine residues to the N-hydroxysuccinyl esters of acrylic and cinnamic acids
Sadofsky, Laura R.,Boa, Andrew N.,Maher, Sarah A.,Birrell, Mark A.,Belvisi, Maria G.,Morice, Alyn H.
, p. 30 - 36 (2011)
The nociceptor TRPA1 is thought to be ac...
A Catalyst-Free Synthesis of Fused Perfluoroalkylated 2,3-Dihydroisoxazoles via Oxa-Michael-Aldol Annulation
Zhou, Wei,Yao, Lan,Liu, Yongxiurong,Shen, Lichun,Chen, Jie,Deng, Hongmei,Shao, Min,Zhang, Hui,Tang, Xiaojun,Cao, Weiguo
supporting information, p. 429 - 438 (2021/10/01)
A novel synthesis of fused perfluoroalky...
DLL3-TARGETING MULTISPECIFIC ANTIGEN-BINDING MOLECULES AND USES THEREOF
-
, (2021/10/02)
The disclosure provides multispecific an...
A METHOD FOR PREPARING N-SUBSTITUTED SUCCINIMIDE
-
Paragraph 0089; 0091-0093; 0095-0108; 0113, (2021/10/27)
N -substituted succinimides are disclose...
Synthesis method of N-(benzyloxycarbonyloxy)succinimide
-
Paragraph 0005; 0008; 0012; 0015, (2020/05/05)
The invention relates to a synthesis met...
6066-82-6 Process route
-
-
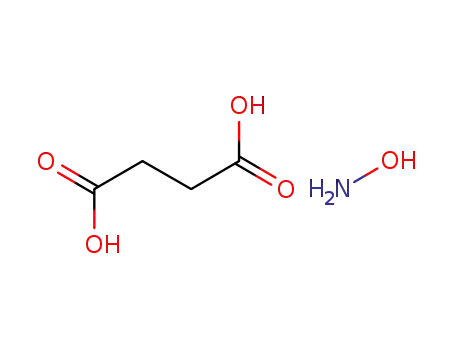
869669-06-7
hydroxylamine succinic acid salt

-
-
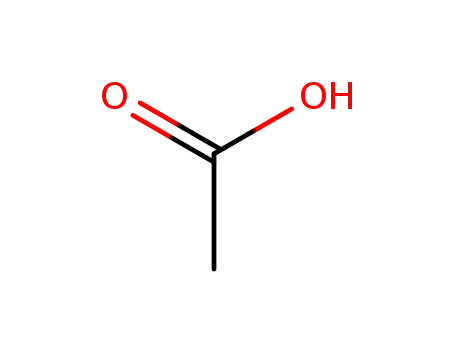
64-19-7,77671-22-8
acetic acid

-
-
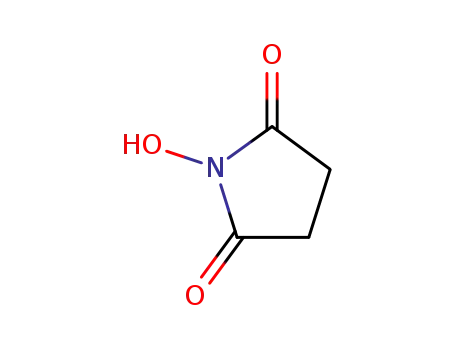
6066-82-6
1-hydroxy-pyrrolidine-2,5-dione

-
-
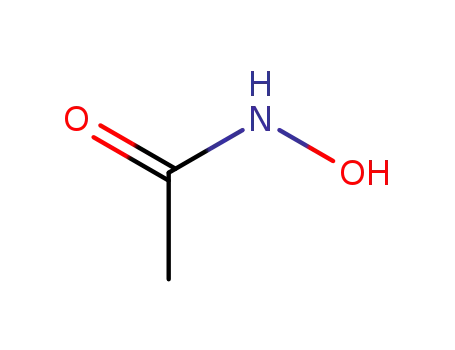
546-88-3
acetylhydroxamic acid
| Conditions | Yield |
|---|---|
|
for 10 - 15h;
Product distribution / selectivity;
Heating / reflux;
|
54.4% 3.7% |
-
-
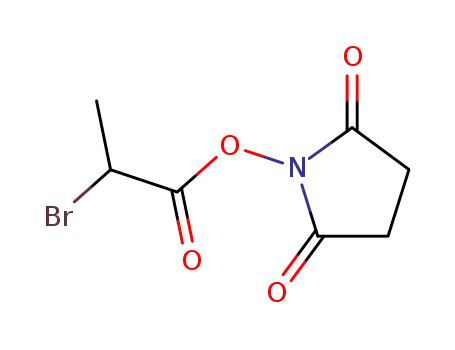
129779-14-2
α-bromopropionyl N-hydroxysuccinimide ester

-

-
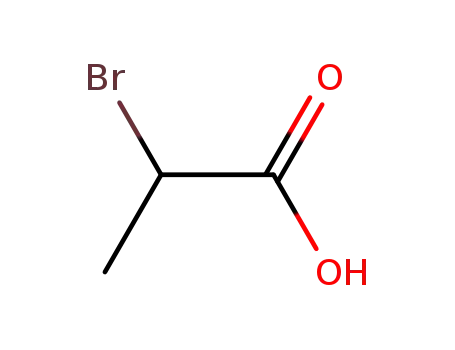
6066-82-6
1-hydroxy-pyrrolidine-2,5-dione

-
-
598-72-1,10327-08-9
2-Bromopropionic acid
| Conditions | Yield |
|---|---|
|
With
water;
|
6066-82-6 Upstream products
-
108-30-5
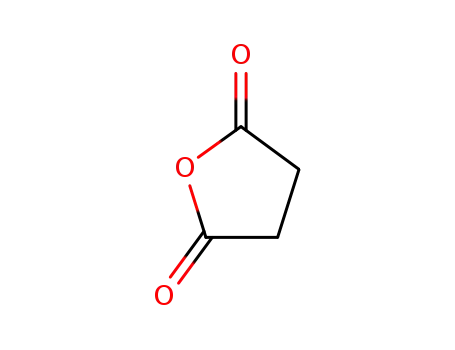
succinic acid anhydride
-
4743-99-1
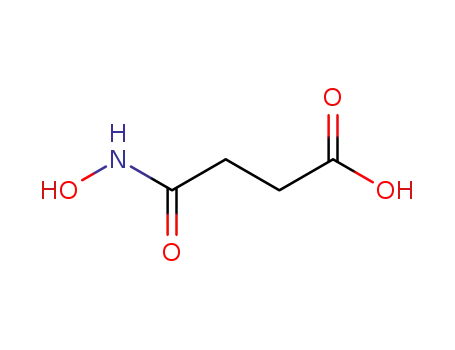
N-hydroxysuccinimide
-
63593-23-7
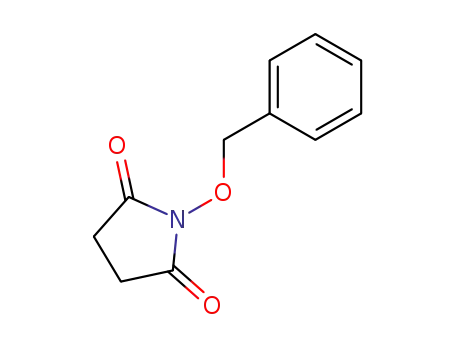
1-(benzyloxy)pyrrolidine-2,5-dione
-
30364-57-9
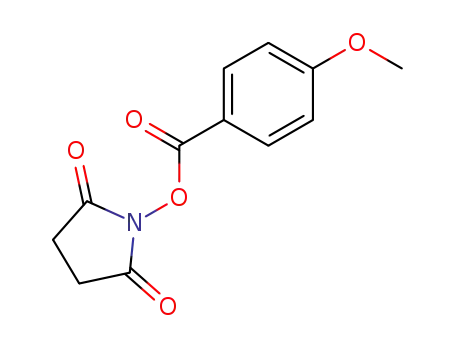
4-methoxybenzoic acid 2,5-dioxo-1-pyrrolidinyl ester
6066-82-6 Downstream products
-
14464-29-0
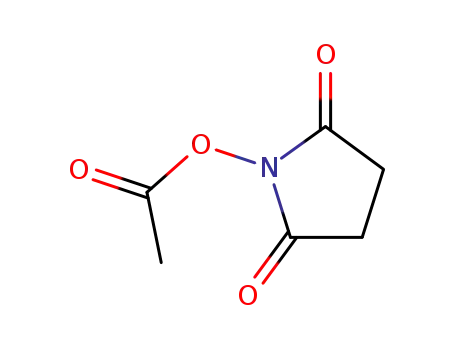
N-acetoxysuccinimide
-
64419-23-4
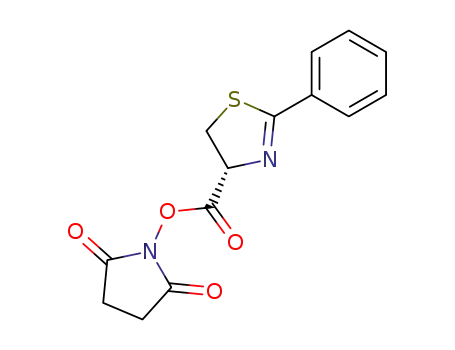
N-((R)-2-phenyl-4,5-dihydro-thiazole-4-carbonyloxy)-succinimide
-
55610-40-7
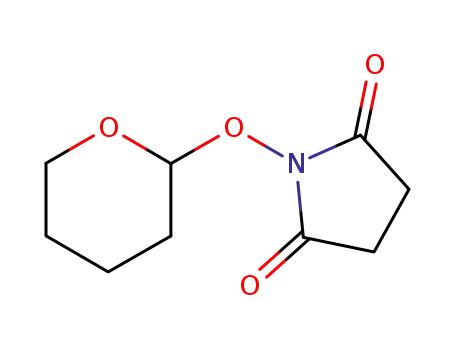
N-(tetrahydropyranyloxy)succinimide
-
71875-81-5
4-(N-maleimidomethyl)cyclohexanecarboxylic acid N-hydroxysuccinimide ester
Relevant Products
-
Tesamorelin
CAS:218949-48-5
-
Talc
CAS:14807-96-6
-
Histidine
CAS:71-00-1