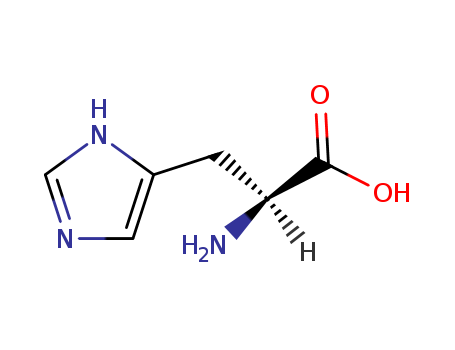
71-00-1
- Product Name:Histidine
- Molecular Formula:C6H9N3O2
- Purity:99%
- Molecular Weight:155.156
Product Details;
CasNo: 71-00-1
Molecular Formula: C6H9N3O2
Appearance: white crystalline powder
Factory Supply industrial standard Histidine 71-00-1 In Stock
- Molecular Formula:C6H9N3O2
- Molecular Weight:155.156
- Appearance/Colour:white crystalline powder
- Melting Point:282 °C (dec.)(lit.)
- Refractive Index:13 ° (C=11, 6mol/L HCl)
- Boiling Point:458.9 °C at 760 mmHg
- PKA:1.91±0.10(Predicted)
- Flash Point:231.3 °C
- PSA:92.00000
- Density:1.423 g/cm3
- LogP:0.06440
Histidine(Cas 71-00-1) Usage
|
Chemical Description |
Histidine is an alpha-amino acid containing an isopyrazole ring. It is a constituent amino acid of body proteins and is found in some functional proteins such as histones and hemoglobin. |
|
Physiological Functions |
Histidine is a natural chelating agent and is involved in the structure and function of many enzymes. |
|
Molecular Interactions |
The versatility of histidine in molecular interactions arises from its unique molecular structure. Histidine's imidazole side chain can engage in various molecular interactions, including cation-π interaction, π-π stacking interaction, hydrogen-π interaction, coordinate bond interaction, and hydrogen bond interaction. These interactions contribute to histidine's role in protein structure, enzymatic function, and other physiological processes. |
|
Nutritional and Therapeutic Uses |
Histidine is considered an essential amino acid for young children but non-essential for adults. It has been used as a nutritional supplement in various conditions such as rheumatoid arthritis, anaemia in chronic renal failure, fatigue during exercise, ageing-related disorders, metabolic syndrome, atopic dermatitis, ulcers, inflammatory bowel diseases, ocular diseases, and neurological disorders. |
|
General Description |
Histidine is an essential amino acid that is crucial for the synthesis of proteins in the human body. It plays a key role in the growth and repair of tissues, as well as in the maintenance of the myelin sheath that protects nerve cells. Additionally, histidine is a precursor for the synthesis of histamine, which is involved in various physiological processes such as digestion, immune response, and inflammation. It also acts as a metal chelator, binding with heavy metals to aid in their excretion from the body. Histidine is found in various protein-rich foods such as meat, fish, dairy products, and certain grains, making it an important nutrient for overall health and well-being. |
InChI:InChI:1S/C6H9N3O2/c7-5(6(10)11)1-4-2-8-3-9-4/h2-3,5H,1,7H2,(H,8,9)(H,10,11)
71-00-1 Relevant articles
An unprecedented trans-oriented product from the cleavage of a dipeptide.
Saha, Manas K,Bernal, Ivan
, p. 612 - 613 (2003)
An unusual trans cleavage reaction was o...
High-throughput screening for the asymmetric transformation reaction of L-histidine to D-histidine by capillary array electrophoresis
Wang, Jun,Liu, Kaiying,Sun, Guangming,Bai, Jiling,Wang, Li
, p. 901 - 904 (2006)
Asymmetric transformation reaction of L-...
Direct monitoring of biocatalytic deacetylation of amino acid substrates by1H NMR reveals fine details of substrate specificity
De Cesare, Silvia,McKenna, Catherine A.,Mulholland, Nicholas,Murray, Lorna,Bella, Juraj,Campopiano, Dominic J.
supporting information, p. 4904 - 4909 (2021/06/16)
Amino acids are key synthetic building b...
Fluorinated S-Adenosylmethionine as a Reagent for Enzyme-Catalyzed Fluoromethylation
Bauer, Carsten,Liao, Cangsong,Peng, Jiaming,Seebeck, Florian P.
, p. 27178 - 27183 (2021/11/16)
Strategic replacement of protons with fl...
Safe and Effective Method of Treating Ulcerative Colitis with Anti-IL12/IL23 Antibody
-
, (2020/04/10)
Described are methods and compositions f...
A green-by-design bioprocess for l-carnosine production integrating enzymatic synthesis with membrane separation
Yin, Dong-Ya,Pan, Jiang,Zhu, Jie,Liu, You-Yan,Xu, Jian-He
, p. 5971 - 5978 (2019/11/14)
l-Carnosine (l-Car, β-alanyl-l-histidine...
71-00-1 Process route
-
Hizikia fusiformis lectine
-
Hizikia fusiformis lectine

-
-
56-41-7,25191-17-7,18875-37-1
L-alanin

-
-
52-90-4
L-Cysteine

-
-
72-19-5,134357-96-3,7013-32-3,82822-12-6
L-threonine

-
-
61-90-5,21675-61-6,25248-98-0,70-45-1
L-leucine

-
-
73-32-5,959215-79-3,18875-42-8
L-isoleucine

-
-
63-68-3,26062-47-5,58576-49-1
L-methionine

-
-
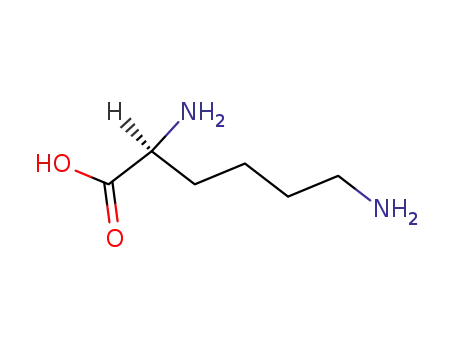
56-87-1,3506-25-0
L-lysine

-
-
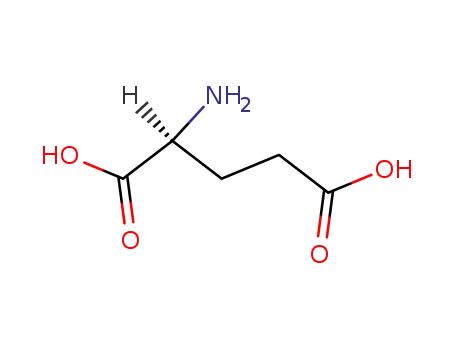
56-86-0,21675-62-7,23009-64-5,25104-13-6,84960-48-5,25513-46-6
L-glutamic acid

-
-
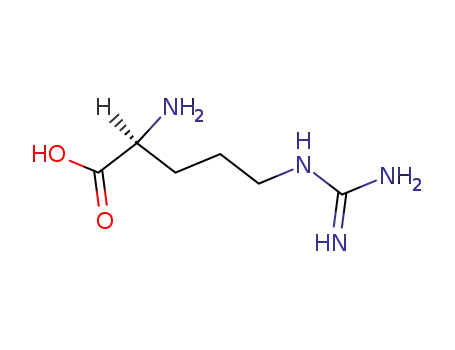
74-79-3,25212-18-4
L-arginine

-
-
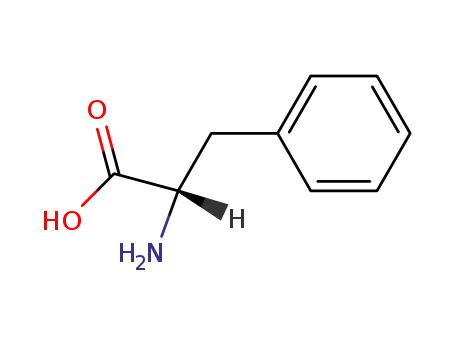
63-91-2,25191-15-5,658-69-5,67675-33-6
L-phenylalanine

-

-
60-18-4,18875-48-4,25619-78-7,30704-25-7
L-tyrosine

-
-
147-85-3,25191-13-3,37159-97-0,4305-67-3,4607-28-7
L-proline

-
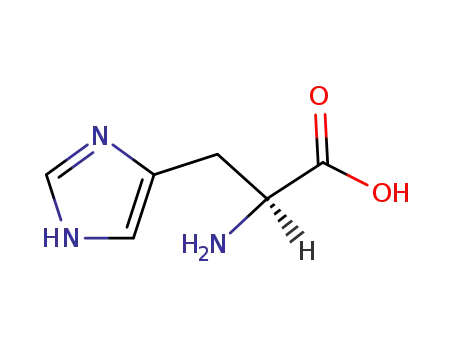
-
71-00-1
L-histidine
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride;
In
water;
at 100 ℃;
for 24h;
Inert atmosphere;
Sealed tube;
|
-
type I collagen from Bester sturgeon scales
-
type I collagen from Bester sturgeon scales

-

-
56-41-7,25191-17-7,18875-37-1
L-alanin

-
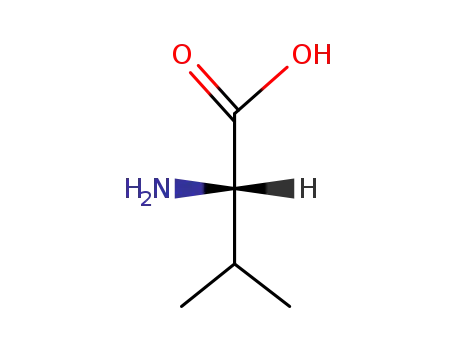
-
72-18-4,25609-85-2,7004-03-7,921-10-8
L-valine

-
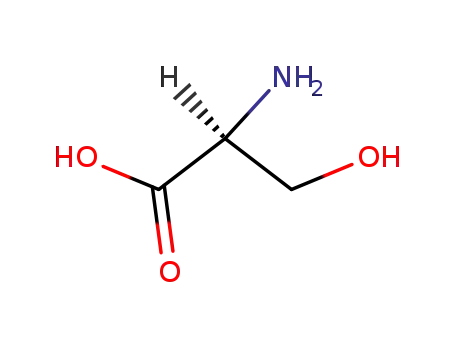
-
56-45-1,83245-15-2
L-serin

-

-
72-19-5,134357-96-3,7013-32-3,82822-12-6
L-threonine

-

-
61-90-5,21675-61-6,25248-98-0,70-45-1
L-leucine

-

-
73-32-5,959215-79-3,18875-42-8
L-isoleucine

-

-
63-68-3,26062-47-5,58576-49-1
L-methionine

-

-
56-87-1,3506-25-0
L-lysine

-
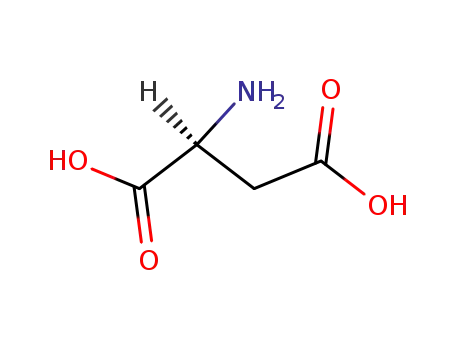
-
56-84-8,25608-40-6,27881-03-4,32505-46-7,52526-39-3
L-Aspartic acid

-
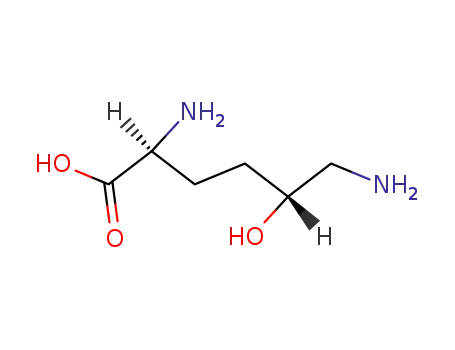
-
504-91-6,1190-94-9,3506-26-1,6000-08-4,18899-29-1,18899-30-4,18899-31-5,52153-41-0,78088-29-6,28902-93-4
(5R)-5-hydroxy-L-lysine

-

-
74-79-3,25212-18-4
L-arginine

-
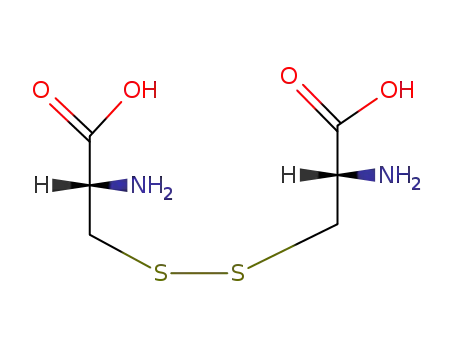
-
349-46-2
S,S-cystine

-

-
63-91-2,25191-15-5,658-69-5,67675-33-6
L-phenylalanine

-

-
60-18-4,18875-48-4,25619-78-7,30704-25-7
L-tyrosine

-
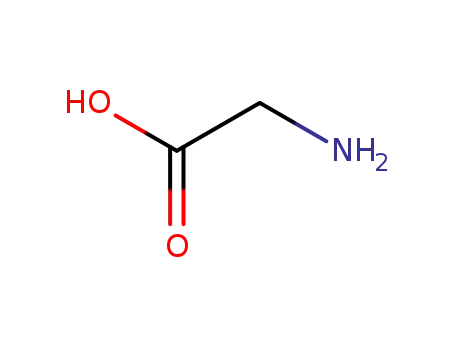
-
56-40-6,18875-39-3,25718-94-9
glycine

-
-
147-85-3,25191-13-3,37159-97-0,4305-67-3,4607-28-7
L-proline

-
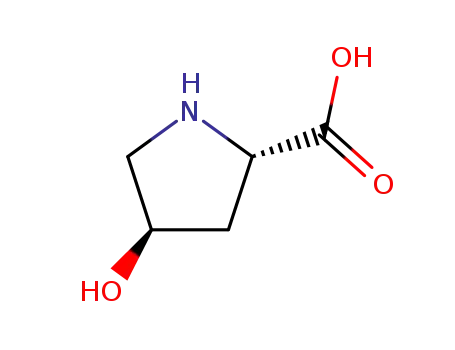
-
51-35-4,30724-02-8
4R-4-hydroxyproline

-

-
71-00-1
L-histidine
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; water;
at 110 ℃;
for 24h;
|
71-00-1 Upstream products
-
7664-41-7
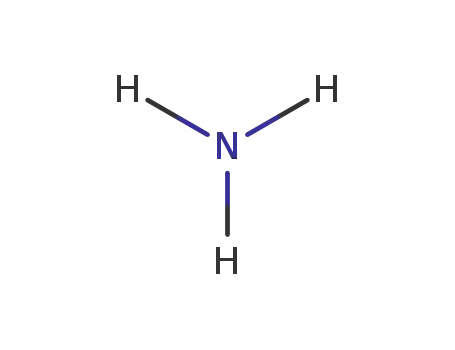
ammonia
-
6630-42-8
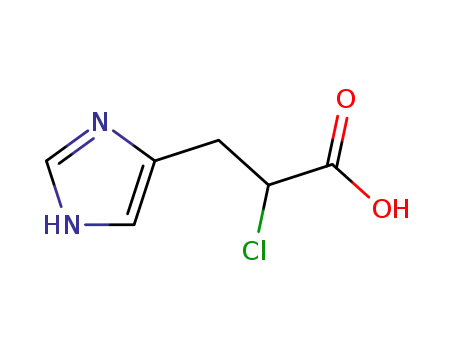
2-chloro-3-(1H-imidazol-4-yl)propanoic acid
-
7664-93-9
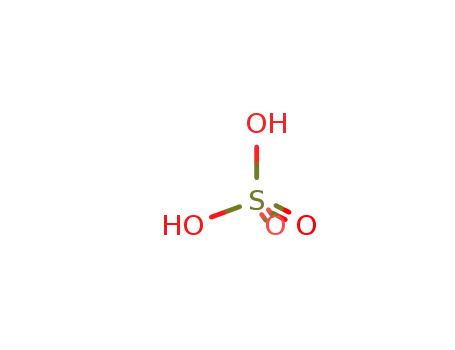
sulfuric acid
-
305-84-0
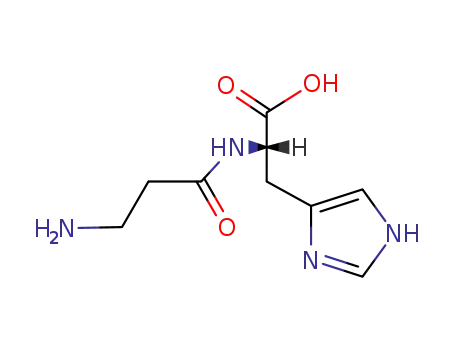
Carnosine
71-00-1 Downstream products
-
15191-21-6
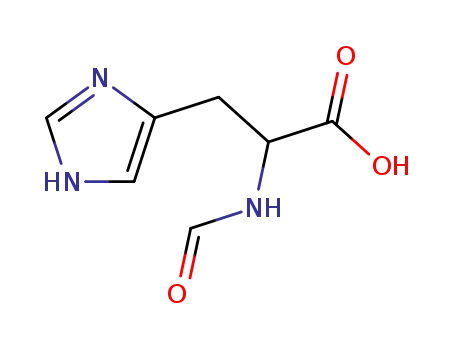
Nα-formyl-histidine
-
133101-94-7
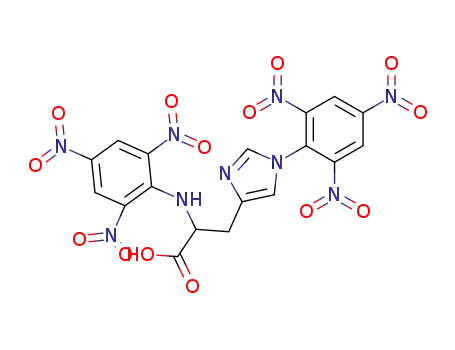
1,Nα-dipicryl-histidine
-
18502-05-1
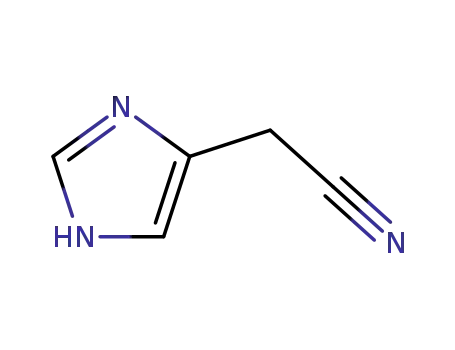
4-cyanomethylimidazole
-
288-32-4
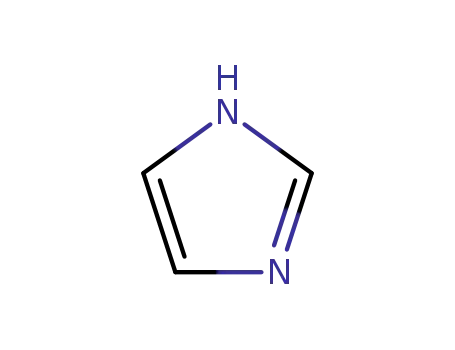
1H-imidazole
Relevant Products
-
Tesamorelin
CAS:218949-48-5
-
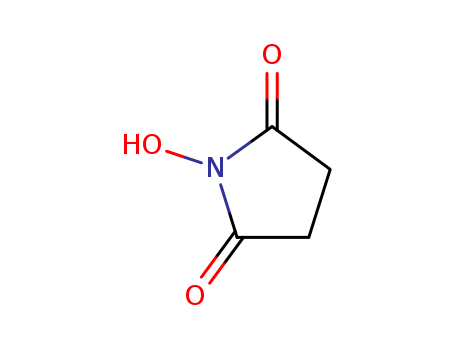
N-Hydroxysuccinimide
CAS:6066-82-6
-
Rosin
CAS:8050-09-7