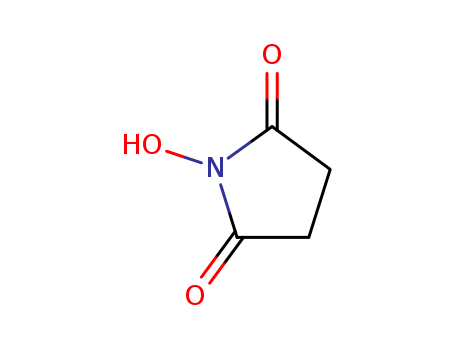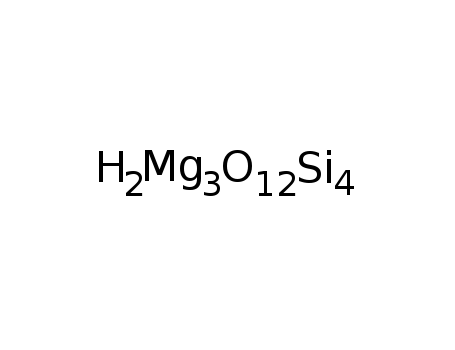
14807-96-6
- Product Name:Talc
- Molecular Formula:H2Mg3O12Si4
- Purity:99%
- Molecular Weight:379.266
Product Details;
CasNo: 14807-96-6
Molecular Formula: H2Mg3O12Si4
Appearance: white to almost white micro fine powder
Factory Export Top Purity Talc 14807-96-6 In Stock
- Molecular Formula:H2Mg3O12Si4
- Molecular Weight:379.266
- Appearance/Colour:white to almost white micro fine powder
- Vapor Pressure:0Pa at 25℃
- Melting Point:800 °C
- PSA:197.00000
- Density:2.7-2.8 g/cm3
- LogP:-2.89430
Talc(Cas 14807-96-6) Usage
|
History |
Talc, soapstone, and steatite have been used by humans as raw materials since prehistoric times. Molds carved from soapstone were used in the Bronze Age and early Iron Age for casting weapons and tools. In the Mediterranean cultures of the classical period, stone carvings were made from soapstone, and talc was used for treating wounds and in the production of cosmetic powder. In ancient Rome, women used large amounts of powder and rouge. The properties of talc, especially its characteristic greasy feel, were described by Pliny the Elder. The old Arabic word talq, which indicates its greasy nature, gave its name to the mineral. In 1550, Catherin de Medici made it once again fashionable to use facial makeup in the form of powdered talc colored by the addition of pigments, a fashion that found innumerable imitators and has continued without interruption until today. |
|
Production Methods |
Talc is a naturally occurring hydropolysilicate mineral found in many parts of the world including Australia, China, Italy, India, France, and the USA. The purity of talc varies depending on the country of origin. For example, Italian types are reported to contain calcium silicate as the contaminant; Indian types contain aluminum and iron oxides; French types contain aluminum oxide; and American types contain calcium carbonate (California), iron oxide (Montana), aluminum and iron oxides (North Carolina), or aluminum oxide (Alabama). Naturally occurring talc is mined and pulverized before being subjected to flotation processes to remove various impurities such as asbestos (tremolite); carbon; dolomite; iron oxide; and various other magnesium and carbonate minerals. Following this process, the talc is finely powdered, treated with dilute hydrochloric acid, washed with water, and then dried. The processing variables of agglomerated talc strongly influence its physical characteristics. |
|
Air & Water Reactions |
Insoluble in water. |
|
Reactivity Profile |
Talc has low reactivity. |
|
Health Hazard |
Pure talc is toxicologically harmless. However, where there are high concentrations of dust in the air, face masks should be worn. If the talc contains detectable amounts of asbestos or asbestos minerals, an MAK value of 2.0 mg/m3 applies. Talc is a nontoxic, inert substance or raw material, but it can contaminate wounds and if inhaled it can cause lung irritations. |
|
Fire Hazard |
Literature sources indicate that Talc is nonflammable. |
|
Flammability and Explosibility |
Notclassified |
|
Pharmaceutical Applications |
Talc was once widely used in oral solid dosage formulations as a lubricant and diluent, although today it is less commonly used. However, it is widely used as a dissolution retardant in the development of controlled-release products. Talc is also used as a lubricant in tablet formulations; in a novel powder coating for extended-release pellets; and as an adsorbant. In topical preparations, talc is used as a dusting powder, although it should not be used to dust surgical gloves. Talc is a natural material; it may therefore frequently contain microorganisms and should be sterilized when used as a dusting powder. Talc is additionally used to clarify liquids and is also used in cosmetics and food products, mainly for its lubricant properties. |
|
Safety Profile |
The talc with less than 1 percent asbestos is regarded as a nuisance dust. Talc with greater percentage of asbestos may be a human carcinogen. A human skin irritant. Prolonged or repeated exposure can produce a form of pulmonary fibrosis (talc pneumoconiosis) which may be due to asbestos content. Questionable carcinogen with experimental tumorigenic data. A common air contaminant. |
|
Safety |
Talc is used mainly in tablet and capsule formulations. Talc is not absorbed systemically following oral ingestion and is therefore regarded as an essentially nontoxic material. However, intranasal or intravenous abuse of products containing talc can cause granulomas in body tissues, particularly the lungs. Contamination of wounds or body cavities with talc may also cause granulomas; therefore, it should not be used to dust surgical gloves. Inhalation of talc causes irritation and may cause severe respiratory distress in infants. Although talc has been extensively investigated for its carcinogenic potential, and it has been suggested that there is an increased risk of ovarian cancer in women using talc, the evidence is inconclusive. However, talc contaminated with asbestos has been proved to be carcinogenic in humans, and asbestos-free grades should therefore be used in pharmaceutical products. Also, long-term toxic effects of talc contaminated with large quantities of hexachlorophene caused serious irreversible neurotoxicity in infants accidentally exposed to the substance. |
|
Carcinogenicity |
In vitro assay of a number of respirable talc specimens of high purity demonstrated a modest but consistent cytotoxicity to macrophages; the investigators conclude that the talcs would be expected to be slightly fibrogenic in vivo. |
|
storage |
Talc is a stable material and may be sterilized by heating at 160°C for not less than 1 hour. It may also be sterilized by exposure to ethylene oxide or gamma irradiation. Talc should be stored in a well-closed container in a cool, dry place. |
|
Toxicity evaluation |
The mechanism by which the talc is toxic is largely physical in its function, impairing organ function by inhibiting necessary movement or transfer of material. Pulmonary responses can occur through production of microemboli and granuloma formation. |
|
Incompatibilities |
Incompatible with quaternary ammonium compounds. |
|
Regulatory Status |
Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (buccal tablets; oral capsules and tablets; rectal and topical preparations). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients. |
|
Physical Properties |
White to grayish-white powder. |
|
Chemical Composition |
Primarily composed of magnesium, silicon, oxygen, and hydrogen. |
|
Characteristics |
Very fine crystalline powder with an unctuous texture. |
|
Environmental and Health Considerations |
Unlikely to have adverse environmental effects or be a concern to human health. Doesn't bioaccumulate and has a low potential to adsorb to soil or sediment. |
|
Definition |
Talc, a hydrated magnesium sulfate, is the primary component of most face powders (eye shadows and blushers). In some products, talc makes up to 70% of the formulation. Cosmetic talc should be white, free of asbestos, should have high spreadability or slip, with low coverage. Particle size is acceptable if the material passes through a 200 mesh sieve. Micronized talc is generally lighter and fluffier but less smooth on the skin than regular grades. Typically talc products are sterilized using gamma irradiation. Cosmetic talcs are mined in Italy, France, Norway, India, Spain, China, Egypt, Japan, and the United States. Talc is fairly hydrophobic, although treatments are used to enhance its texture. |
|
General Description |
Odorless white to grayish-white very fine crystalline powder (unctuous). Readily adheres to the skin. Nonflammable, noncombustible, and nontoxic. |
InChI:InChI=1/3Mg.4O2Si.H2O.3O/c;;;4*1-3-2;;;;/h;;;;;;;1H2;;;/r3MgO.4O2Si.H2O/c3*1-2;4*1-3-2;/h;;;;;;;1H2
14807-96-6 Relevant articles
Changes to the triaxial composition of the hydrated phases (CaO/Al 2O3/SiO2) in the metakaolin/lime system
Garcia Gimenez, Rosario,Rodriguez, Olga,Vigil De La Villa, Raquel,Frias, Moises
, p. 1118 - 1122 (2012)
This study examines the composition of c...
Studies of the Rate of the Formation of Talc under Hydrothermal Conditions at 400-460 deg C
Muraishi, Haruto
, p. 1071 - 1076 (1988)
The rate of the formation of talc from b...
REACTION OF MAGNESIUM HYDROXIDE WITH SOLUBLE SILICA UNDER HYDROTHERMAL CONDITIONS BELOW THE CRITICAL TEMPERATURE.
Muraishi
, p. 878 - 883 (1981)
The mechanism of the synthetic reaction ...
Synthesis of magnesium silicate by heat treatment of sols and mechanical activation of solid components
Dudkin,Vasyutin
, p. 751 - 755 (2011/09/14)
A layered magnesium silicate, an analog ...
Pharmaceutical composition comprising N-[2-(Diethylamino)ethyl]-5-[(5-fluoro-1,2-dihydro-2-oxo-3H-indol-3-ylidene) methyl]-2,4-dimethyl-1H-pyrrole-3-carboxamide
-
, (2010/01/07)
The present invention relates to a pharm...
ANTIPSYCHOTIC BENZOTHIOPYRANYLAMINES
-
, (2008/06/13)
Disclosed are 3,4-dihydro-2H-1-benzothio...
14807-96-6 Process route

-
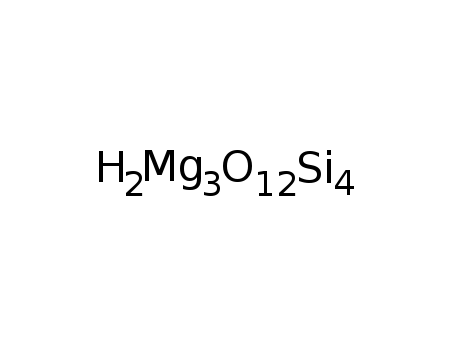
-
14807-96-6
magnesium silicate

-
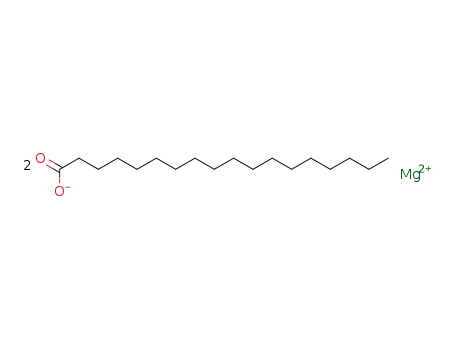
-
557-04-0
magnesium stearate
| Conditions | Yield |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
-
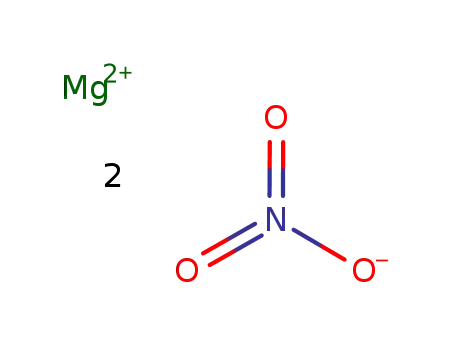
-
13446-18-9
magnesium(II) nitrate

-

-
112926-00-8,7631-86-9
silica gel

-
-
14807-96-6
magnesium silicate
| Conditions | Yield |
|---|---|
|
With
KOH;
In
melt;
Mg(NO3)2 was added to an eutectic melt of KNO3/LiNO3 at 350 °C, followed by a melt of SiO2 in KOH;; the pptd. prod. was either isolated by filtration of the melt through a layer of compressed quartz powder or by leaching the cooled mass with water, alcohol, or acetone;
|
14807-96-6 Upstream products
-
13446-18-9

magnesium(II) nitrate
-
112926-00-8

silica gel
-
7786-30-3

magnesium chloride
14807-96-6 Downstream products
-
7732-18-5
water
-
112926-00-8

silica gel
Relevant Products
-
Tesamorelin
CAS:218949-48-5
-
Carboplatin
CAS:41575-94-4
-
N-Hydroxysuccinimide
CAS:6066-82-6