50-99-7
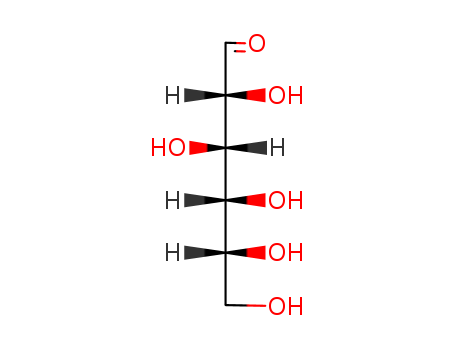
- Product Name:D(+)-Glucose
- Molecular Formula:C6H12O6
- Purity:99%
- Molecular Weight:180.158
Product Details;
CasNo: 50-99-7
Molecular Formula: C6H12O6
Appearance: White crystalline powder
Buy Quality D(+)-Glucose 50-99-7 In Stock with Immediately Delivery
- Molecular Formula:C6H12O6
- Molecular Weight:180.158
- Appearance/Colour:White crystalline powder
- Vapor Pressure:2.59E-13mmHg at 25°C
- Melting Point:150-152 °C(lit.)
- Refractive Index:53 ° (C=10, H2O)
- Boiling Point:527.112 °C at 760 mmHg
- PKA:pKa 12.43(H2O,t = 18,)(Approximate)
- Flash Point:286.664 °C
- PSA:118.22000
- Density:1.581 g/cm3
- LogP:-3.37880
D(+)-Glucose(Cas 50-99-7) Usage
|
History |
D(+)-Glucose?is the most important and predominant monosaccharide found in nature. It was isolated from raisins by Andreas Sigismund Marggraf (1709–1782) in 1747, and in 1838, Jean-Baptiste-André Dumas (1800–1884) adopted the name glucose from the Greek word glycos meaning sweet. Emil Fischer (1852–1919) determined the structure of glucose in the late 19th century. Glucose also goes by the names dextrose (from its ability to rotate polarized light to the right), grape sugar, and blood sugar. The term blood sugar indicates that glucose is the primary sugar dissolved in blood. Glucose’s abundant hydroxyl groups enable extensive hydrogen bonding, and so glucose is highly soluble in water. |
|
Manufacturing Process |
D-Glucose is naturally occurring and is found in fruits and other parts of plants in its free state. It is used therapeutically in fluid and nutrient replacement.Dehydration of Dextrose Monohydrate.1. Dehydration with Fluid-bed DryerDextrose monohydrate was brought in a horizontal-placed turbo-dryer (VOMM, Mailand, Italy). The dehydration occurred at a temperature of between 90° to 150°C in a stream of air of 5 Normalised m3/kg (i.e volume of gas at 0°C and 1 mbar) dextrose and a rotation speed of 1200 min-1.Dehydration of Glucose Syrup (Dextrose Content 96%).A glucose syrup (C*SWEET D 02763 Cerestar) (dry substance ca. 70%) was sprayed at a flow rate of 7 kg/h at 70°C into a Niro FSD pilot plant spray dryer. For powdering ca. 9 kg coarsely milled dried product at a ratio liquid/solid of 1:2 was added. The atomising conditions were as follows:The drying chamber was operated at:The fluid bed was adjusted to: |
|
Biotechnological Production |
The D-configuration of D-isoascorbic acid at C5 allows a short biosynthetic pathway from D-glucose, i.e., its 1,5-glucopyranoside, which is oxidized to D-glucono-1,5-lactone by glucose oxidase followed by oxidation at C2 by D-gluconolactone oxidase. The immediate oxidation product of D-glucono-1,5-lactone by gluconolactone oxidase already has reducing activity on, e.g., 2,6-dichlorphenolindophenol. It is rather stable at pH 4. Upon pH shift, this compound spontaneously converts to D-isoascorbic acid. The unidentified immediate oxidation product could be 2-keto-D-glucono-1,5-lactone, which rearranges via a reversible transesterification reaction to the 1,4-lactone followed by an irreversible enolization to D-isoascorbic acid. The formation of 2-keto-D-gluconic acid as the result of 2-keto-D-glucono-1,5-lactone hydrolysis was not reported. The oxidation of the 1,4-lactone by D-gluconolactone oxidase might also occur to some extent, since D-glucono-1,5-lactone shows a tendency to slowly rearrange to the 1,4-lactone at pH[4and the D-gluconolactone oxidase of Penicillium cyaneofulvum accepts both D-glucono-1,5-lactone and the corresponding 1,4-lactone . This reaction would directly deliver the keto-isomer of D-isoascorbic acid. The sequence of the reactions from D-glucose to D-isoascorbic acid, first oxidation at C1, then oxidation at C2 (C1, C2), is similar to the naturally evolved Asc biosynthesis from L-galactose or L-gulose. Oxidation of D-gluconolactone at C2 is also afforded by pyranose-2-oxidase from Polyporus obtusus. In this reaction both D-isoascorbic acid and 2-keto- D-gluconic acid were obtained in a roughly 1:1 ratio. Obviously, following the natural C1, C2 oxidation sequence, transesterification and (iso)ascorbic acid formation are preferred over hydrolysis and 2-keto sugar acid formation or are at least possible to a significant extent. If the sequence of oxidation reactions is reversed (C2, C1), i.e., D-glucopyranose is first oxidized by pyranose-2-oxidase to D-glucosone followed by glucose oxidase treatment, 2-keto-D-gluconate was reported as the only oxidation product. Though not explicitly reported, it is safe to assume that the later oxidation occurs with 2-keto-D-gluco-1,5-pyranose and delivers as the immediate reaction product 2-keto-D-glucono-1,5-lactone, which hydrolyzes affording 2-keto-D-gluconate. It is unclear why the spontaneous follow-up reaction of 2-keto-D-glucono-1,5-lactone delivers, at least to some extent, D-isoascorbic acid if obtained according to the C1, C2 reaction sequence, but only 2-keto-D-gluconate if obtained by the C2, C1 oxidation sequence. |
|
Air & Water Reactions |
Water soluble. |
|
Reactivity Profile |
A weak reducing agent. |
|
Health Hazard |
No toxicity |
|
Biochem/physiol Actions |
Glycogen phosphorylase, muscle associated (PYGM), is an important contributor to glycogenolysis. Down regulation of PYGM gene is observed in schizophrenia. Mutation in PYGM leads to McArdle disease, a glycogen storage disorder. The PYGM gene is significantly associated with energy production. |
|
Safety Profile |
Mildly toxic by ingest ion. An experimental teratogen. Experi mental reproductive effects. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. Potentially explosive reaction with potassium nitrate + sodium peroxide when heated in a sealed container. Uxtures with alkali release carbon monoxide when heated. When heated to decomposition it emits acrid smoke and irritating fumes. |
|
Purification Methods |
Crystallise -D-glucose from hot glacial acetic acid or pyridine. Traces of solvent are removed by drying in a vacuum oven at 75o for >3hours. [Gottfried Adv Carbohydr Chem 5 127 1950, Kjaer & Lindberg Acta Chem Scand 1 3 1713 1959, Whistler & Miller Methods in Carbohydrate Chemistry I 1301962, Academic Press, Beilstein 1 IV 4306.] [For equilibrium forms see Angyal Adv Carbohydr Chem 42 15 1984, Angyal & Pickles Aust J Chem 25 1711 1972.] |
|
General Description |
D(+)-Glucose can be selectively detected in aqueous solutions through a fluorescence turn-on mechanism involving its enzymatic oxidation by glucose oxidase (GOx) to produce hydrogen peroxide (H2O2), which then reacts with a modified tetraphenylethylene (TPE)-based probe. This reaction triggers aggregation-induced emission (AIE), resulting in a measurable fluorescence signal, demonstrating high sensitivity and specificity for D-glucose with a detection limit as low as 3.0 μM. The method is highly selective, showing minimal interference from other sugars or reactive oxygen species. |
|
Definition |
Naturally occurring GLUCOSE belongs to the stereochemical series D and is dextrorotatory, indicated by the symbol (+). Thus the term dextrose is used to indicate D-(+)-glucose. As other stereochemical forms of glucose have no significance in biological systems the term ‘glucose’ is often used interchangeably with dextrose in biology. |
InChI:InChI=1/C6H12O6/c7-1-3(9)5(11)6(12)4(10)2-8/h1,3-6,8-12H,2H2/t3-,4+,5+,6+/m0/s1
50-99-7 Relevant articles
A new secoiridoid glycoside and a new sesquiterpenoid glycoside from Valeriana jatamansi with neuroprotective activity
Tan, Yu-Zhu,Yong, Yan,Dong, Yan-Hong,Wang, Ru-Jing,Li, Hong-Xiang,Zhang, Hai,Guo, Da-Le,Zhang, Shi-Jin,Dong, Xiao-Ping,Xie, Xiao-Fang
, p. 177 - 180 (2016)
A new secoiridoid glycoside, isopatrinio...
Anthraquinone glycosides from Cassia roxburghii and evaluation of its free radical scavenging activity
El-Toumy, Sayed A.,El Souda, Sahar S.,Mohamed, Tahia K.,Brouard, Inaki,Bermejo, Jame
, p. 47 - 51 (2012)
The methanolic extract of the leaves of ...
A new triterpene glycoside from fruit of Phytolacca americana
Getiya,Gabelaya,Mshvildadze,Pichette,Lavoie,Dekanosidze
, p. 764 - 766 (2011)
Glycosides H and I, the structures of wh...
New phenolic glycosides from Polygonum cuspidatum
Jiang, Jian-Shuang,Li, Fu-Shuang,Feng, Zi-Ming,Yang, Ya-Nan,Zhang, Pei-Cheng
, p. 17 - 23 (2020)
Two new isobenzofuranone derivatives, po...
Substrate control through per-O-methylation of cyclodextrin acids
Fenger, Thomas H.,Bols, Mikael
, p. 7769 - 7771 (2010)
Per-O-methylated cyclodextrins containin...
Three new glycosides from Hylocereus undatus
Wu, Xin,Wang, Ying,Huang, Xiao-Jun,Fan, Chun-Lin,Wang, Guo-Cai,Zhang, Xiao-Qi,Zhang, Qin-Wen,Ye, Wen-Cai
, p. 728 - 733 (2011)
Three new glycosides, undatusides A-C (1...
Polysciosides J and K, two new oleanane-type triterpenoid saponins from the leaves of Polyscias fruticosa (L.) harms. cultivating in An Giang Province, Viet Nam
Do, Van Mai,Tran, Cong Luan,Nguyen, Tan Phat
, p. 1250 - 1255 (2020)
For the first time, the phytochemical co...
NMR-Based Investigation of Hydrogen Bonding in a Dihydroanthracen-1(4 H)one from Rubia philippinensis and Its Soluble Epoxide Hydrolase Inhibitory Potential
Oh, Joonseok,Quan, Khong Trong,Lee, Ji Sun,Park, Inwha,Kim, Chung Sub,Ferreira, Daneel,Thuong, Phuong Thien,Kim, Young Ho,Na, Minkyun
, p. 2429 - 2435 (2018)
Hydrogen bonding is a vital feature of a...
A membrane-bound trehalase from Chironomus riparius larvae: Purification and sensitivity to inhibition
Forcella, Matilde,Cardona, Francesca,Goti, Andrea,Parmeggiani, Camilla,Cipolla, Laura,Gregori, Maria,Schirone, Raffaella,Fusi, Paola,Parenti, Paolo
, p. 1186 - 1195 (2010)
A preparation of a membrane-bound trehal...
New cycloartane glycosides from the rhizomes of Cyperus rotundus and their antidepressant activity
Zhou, Zhong-Liu,Lin, San-Qing,Yin, Wen-Qing
, p. 662 - 668 (2016)
Two new cycloartane glycosides, cyprotus...
New grayanol diterpenoid and new phenolic glucoside from the flowers of Pieris formosa
Wang, Wei-Guang,Li, Hong-Mei,Li, Hai-Zhou,Wu, Zhao-Yuan,Li, Rong-Tao
, p. 70 - 75 (2010)
A new grayanol diterpenoid, grayanotoxin...
Acridone alkaloids from the rhizomes of Luvunga scandens (Roxb.) Buch. Ham.
Tran, Nguyen Minh An,Do, Thi Hong Tuoi,Truong, Luu Hong,Le, Dung Tien,Phan, Minh Nhat,Pham, Nguyen Kim Tuyen,Mai, Dinh Tri,Nguyen, Tan Phat
, p. 2176 - 2181 (2019)
The ethyl acetate extract of the rhizome...
Three new phenol compounds from Iris dichotoma PALL
Huang, Long,Ma, Wenhui,Liu, Yanze,Peng, Yong,Xiao, Peigen
, p. 1033 - 1036 (2012)
Three new phenolic compounds, irisdichot...
Antidiabetic ellagitannins from pomegranate flowers: Inhibition of α-glucosidase and lipogenic gene expression
Yuan, Tao,Ding, Yuanqing,Wan, Chunpeng,Li, Liya,Xu, Jialin,Liu, Ke,Slitt, Angela,Ferreira, Daneel,Khan, Ikhlas A.,Seeram, Navindra P.
, p. 5358 - 5361 (2012)
Two new ellagitannins containing a rare ...
New dammarane-type triterpenoid glycosides from Gynostemma burmanicum
Nguyen Phuong, Thao,Nguyen Tien, Dat,Pham, Thanh Binh,Pham, Thanh Ky,Than, Thi Kieu My
, p. 217 - 224 (2020)
The chemical composition of Gynostemma b...
Three new lignan glycosides from the Firmiana simplex
Woo, Kyeong Wan,Park, Jong Eel,Cha, Joon Min,Subedi, Lalita,Kim, Sun Yeou,Lee, Kang Ro
, p. 18 - 22 (2019)
In our quest for structurally intriguing...
Isolation and Some Properties of Sorbitol Oxidase from Streptomyces sp. H-7775
Hiraga, Kazumi,Kitazawa, Mitsunori,Kaneko, Norihisa,Oda, Kohei
, p. 1699 - 1704 (1997)
A sorbitol oxidase (SOX) was found in th...
Six new ergostane-type steroids from king trumpet mushroom (Pleurotus eryngii) and their inhibitory effects on nitric oxide production
Kikuchi, Takashi,Maekawa, Yukina,Tomio, Arisa,Masumoto, Yuki,Yamamoto, Taishi,In, Yasuko,Yamada, Takeshi,Tanaka, Reiko
, p. 9 - 17 (2016)
Six new ergostane-type steroids; (22E)-3...
A grayanotox-9(11)-ene derivative from Rhododendron brachycarpum and its structural assignment via a protocol combining NMR and DP4 plus application
Tuan, Nguyen Quoc,Oh, Joonseok,Park, Hyun Bong,Ferreira, Daneel,Choe, Sanggil,Lee, Juseon,Na, MinKyun
, p. 45 - 50 (2017)
A growing body of evidence points to the...
A new chromene glycoside from tithonia diversifolia
Zhai, Hong-Li,Zhao, Gui-Jun,Yang, Gen-Jin,Sun, He,Yi, Bo,Sun, Lian-Na,Chen, Wan-Sheng,Zheng, Shui-Qing
, p. 198 - 200 (2010)
A new chromene glycoside, 6-acetyl-2,2-d...
Cordycepamides A?E and cordyglycoside A, new alkaloidal and glycoside metabolites from the entomopathogenic fungus Cordyceps sp.
Fan, Wenwen,Li, Erwei,Liu, Xingzhong,Ren, Jinwei,Wang, Wenzhao,Zhang, Yongjie
, (2020)
Five new alkaloidal metabolites cordycep...
New coumarins and monoterpene galloylglycoside from the stem bark of Sapium baccatum
Li, Ting,Wang, Shanshan,Fan, Peihong,Lou, Hongxiang
, p. 435 - 442 (2019)
Sapium baccatum has been traditionally u...
Three new sulfated triterpenoids from the roots of Gypsophila pacifica
Luo, Jian-Guang,Nie, Wei,Kong, Ling-Yi
, p. 529 - 533 (2011)
Three new sulfated triterpenoids (1-3), ...
2β-D-GLUCOPYRANOSYLOXY-2-METHYLPROPANOL IN ACACIA SIEBERANA VAR. WOODII
Brimer, Leon,Christensen, S. Broegger,Nartey, Frederick
, p. 2005 - 2008 (1982)
A new diol glucoside, 2-β-D-glucopyranos...
Structural characterization of cholestane rhamnosides from ornithogalum saundersiae bulbs and their cytotoxic activity against cultured tumor cells
Iguchi, Tomoki,Kuroda, Minpei,Naito, Rei,Watanabe, Tomoyuki,Matsuo, Yukiko,Yokosuka, Akihito,Mimaki, Yoshihiro
, (2017)
Previous phytochemical studies of the bu...
Four New Diterpene Glucosides from Perovskia atriplicifolia
Gao, Lu,Zhou, Jun,Zhu, Le-Yu,Zhang, Juan-Rong,Jing, Yu-Xing,Zhao, Jia-Wen,Huang, Xiang-Zhong,Li, Gan-Peng,Jiang, Zhi-Yong,Xue, Da-Yuan
, (2017)
Four new diterpene glucosides, namely pe...
New Glycosides of Eriodictyol from Dracocephalum palmatum
Olennikov,Chirikova,Kim, Eungyoung,Kim, Sang Woo,Zul’fugarov
, (2018)
Two new glycosides of eriodictyol were i...
Three new monoterpene glycosides from Sibiraea laevigata (L.) Maxim
Mei, Li-Juan,Shao, Yun,Shi, Yan-Ping,Tao, Yan-Duo,Wang, Qi-Lan,Wang, Yan-Ming,Zhao, Jian-Qiang
, p. 176 - 180 (2017)
Three new compounds, 3,7-dimethy-7-metho...
Medicinal flowers. XXXX.1) Structures of dihydroisocoumarin glycosides and inhibitory effects on aldose reducatase from the flowers of Hydrangea macrophylla var. thunbergii
Liu, Jiang,Nakamura, Seikou,Zhuang, Yan,Yoshikawa, Masayuki,Hussein, Ghazi Mohamed Eisa,Matsuo, Kyohei,Matsuda, Hisashi
, p. 655 - 661 (2013)
Six dihydroisocoumarin glycosides, flora...
Caragiside D, a new isoflavone glucoside from caragana conferta
Perveen, Shagufta,Al-Taweel, Areej Mohammad,Khan, Afsar,Fawzy, Ghada Ahmed,Malik, Abdul
, p. 440 - 442 (2014)
Caragiside D (1), a new isoflavone gluco...
The bioassay-guided isolation of antifungal saponins from Hosta plantaginea leaves
Wang, Meng-Yue,Peng, Ying,Peng, Chong-Sheng,Qu, Jiang-Yuan,Li, Xiao-Bo
, p. 501 - 509 (2018)
Four new steroidal saponins hostaside Ⅰ ...
A new flavonoid glycoside and four other chemical constituents from viscum coloratum and their antioxidant activity
Fan, Ronghua,Ma, Yuying,Yuan, Hongxia,Zhang, Yongzhi,Wei, Binbin,Zhao, Yunli,Yu, Zhiguo
, p. 1455 - 1462 (2014)
A new flavonoid glycoside, identified as...
Isolation, identification and antioxidative capacity of water-soluble phenylpropanoid compounds from Rhodiola crenulata
Chen, Danjun,Fan, Junting,Wang, Peng,Zhu, Lanying,Jin, Yang,Peng, Yan,Du, Shuhu
, p. 2126 - 2133 (2012)
Six water-soluble phenylpropanoid compou...
Enzymatic and structural characterization of hydrolysis of gibberellin A4 glucosyl ester by a rice β-D-glucosidase
Hua, Yanling,Sansenya, Sompong,Saetang, Chiraporn,Wakuta, Shinji,Cairns, James R. Ketudat
, p. 39 - 48 (2013)
In order to identify a rice gibberellin ...
Four new cytotoxic oligosaccharidic derivatives of 12-oleanene from Lysimachia heterogenea Klatt
Huang, Xin-an,Liang, Yong-ju,Cai, Xiao-ling,Feng, Xiao-quan,Zhang, Chuan-hai,Fu, Li-wu,Deng, Wen-di
, p. 6515 - 6518 (2009)
Cytotoxicity-guided phytochemical analys...
Novel monoterpenoid indole alkaloids from Melodinus yunnanensis
Zhang, Bing-Jie,Liu, Cheng,Bao, Mei-Fen,Zhong, Xiu-Hong,Ni, Ling,Wu, Jing,Cai, Xiang-Hai
, p. 5821 - 5826 (2017)
Five monoterpenoid indole alkaloids, nam...
Two new sesquiterpenoid glycosides from the stems of Zanthoxylum armatum DC
Liu, Ya-Lin,Gao, Liang-Liang,Song, Tong-Tong,Guo, Tao,Chang, Jun
, p. 3036 - 3041 (2020)
Two new sesquiterpenoid glycosides as di...
Micellar catalysis on pentavalent vanadium ion oxidation of D-sorbitol in aqueous acid media: A kinetic study
Saha, Bidyut,Chowdhury, Kiran M.,Mandal, Jayashree
, p. 1321 - 1328 (2008)
Vanadium(V) oxidation of D-sorbitol show...
Depolymerization of cellulosic feedstocks using magnetically separable functionalized graphene oxide
Verma, Deepak,Tiwari, Rashmi,Sinha, Anil Kumar
, p. 13265 - 13272 (2013)
Hydrolysis of cellulose into saccharides...
Xanthone glycosides from swertia bimaculata with α -glucosidase inhibitory activity
Yue, Yao-Dong,Zhang, Yu-Tang,Liu, Zhao-Xia,Min, Qiu-Xia,Wan, Luo-Sheng,Wang, Yong-Long,Xiao, Zuo-Qi,Chen, Jia-Chun
, p. 502 - 508 (2014)
Seven new xanthone glycosides (1-7) were...
New ecdysteroid and ecdysteroid glycosides from the roots of Serratula chinensis
Zhang, Zi-Yue,Yang, Wei-Qun,Fan, Chun-Lin,Zhao, Hui-Nan,Huang, Xiao-Jun,Wang, Ying,Ye, Wen-Cai
, p. 208 - 214 (2017)
Three new ecdysteroid glycosides (1–3) a...
The study of factors influencing the depolymerisation of cellulose using a solid catalyst in ionic liquids
Watanabe, Hisanori
, p. 1168 - 1171 (2010)
Cellulose is the most abundant biomass i...
Complete assignments of 1H and 13C NMR spectroscopic data for three new stigmastane glycosides from Vernonia cumingiana
Suo, Maorong,Yang, Junshan
, p. 179 - 183 (2009)
Three new steroidal saponins, Vernoniosi...
Three new secoiridoid glycosides from the rhizomes and roots of Gentiana scabra and their anti-inflammatory activities
Li, Wei,Zhou, Wei,Kim, Sohyun,Koo, Jung-Eun,Kim, Yuna,Koh, Young-Sang,Shim, Sang Hee,Ma, Jin Yeul,Ho Kim, Young
, p. 1920 - 1927 (2015)
Three new (1-3) and 17 known (4-20) irid...
Use of electrospray ionization ion-trap tandem mass spectrometry and principal component analysis to directly distinguish monosaccharides
Xia, Bing,Zhou, Yan,Liu, Xin,Xiao, Juan,Liu, Qing,Gu, Yucheng,Ding, Lisheng
, p. 1259 - 1264 (2012)
RATIONALE Carbohydrates are good source ...
Three new lignan glycosides with IL-6 inhibitory activity from Akebia quinata
Jin, Hong-Guang,Kim, A. Ryun,Ko, Hae Ju,Lee, Sang Kook,Woo, Eun-Rhan
, p. 288 - 293 (2014)
Three new lignan glycosides, akeqintosid...
Flavonoids and terpenoids with PTP-1B inhibitory properties from the infusion of salvia amarissima ortega
González-Andrade, Martín,Mata, Rachel,Pérez-Vásquez, Araceli,Rangel-Grimaldo, Manuel,Rivero-Cruz, Isabel,Salinas-Arellano, Eric,Torres-Colin, Rafael
, (2020)
An infusion prepared from the aerial par...
Purification and characterization of a novel GH1 beta-glucosidase from Jeotgalibacillus malaysiensis
Liew, Kok Jun,Lim, Lily,Woo, Hui Ying,Chan, Kok-Gan,Shamsir, Mohd Shahir,Goh, Kian Mau
, p. 1094 - 1102 (2018)
Beta-glucosidase (BGL) is an important i...
A new megastigmane diglycoside from litsea glutinosa (Lour.) C. B. Rob.
Wang, Yun-Song,Liao, Zhen,Li, Yan,Huang, Rong,Zhang, Hong-Bing,Yang, Jing-Hua
, p. 2234 - 2238 (2011)
Phytochemical study on the leaves and tw...
Two new dammarane-type triterpenoid saponins from notoginseng medicinal fungal substance
Xu, Wei,Liu, Xin,Liu, Xiao-Li,Jia, Ai-Ling,Wang, Xin-Wen,Qiu, Zhi-Dong
, p. 1138 - 1142 (2016)
Two new dammarane-type triterpenoid sapo...
Two new glycosides from Dianella ensifolia (L.) DC
Fan, Miao-Yin,Liu, Bing-Rui,Yang, Fan,Zhang, Pu-Zhao
, p. 18 - 20 (2021/11/11)
The Dianella genus includes approximatel...
Long-chain fatty acid acylated derivatives of isoflavone glycosides from the rhizomes of Iris domestica
Li, Jiayuan,Liu, Yanfei,Ni, Gang,Wang, Renzhong,Yu, Dequan
, (2021/11/01)
Six undescribed long-chain fatty acid es...
New glycosides from the leaves and rattan stems of Schisandra chinensis
Bao, TeRiGen,Fu, ChunWang,Jia, JingMing,Liu, LuQi,Wang, AnHua,Yang, YongCheng
, p. 21 - 23 (2021/11/11)
Two new glycosides, vanillic acid 4-O-β-...
Iridoid glucosides from the leaves of Vitex negundo var. cannabifolia
Huo, Huixia,Li, Jun,Li, Manman,Liang, Naiyun,Ma, Jiale,Song, Yuelin,Sun, Jing,Tu, Pengfei,Wang, Rongye,Zhao, Yunfang,Zheng, Jiao
, p. 56 - 62 (2021/11/30)
Five new iridoid glucosides, cannabifoli...
50-99-7 Process route
-
-
2492-87-7
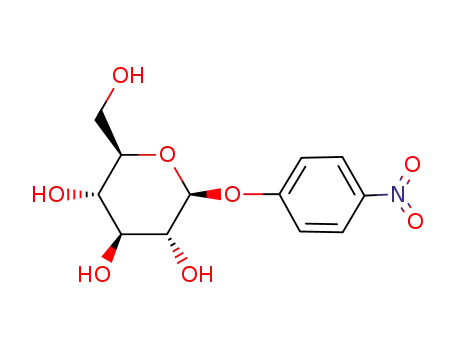
4-nitrophenyl-β-D-glucoside

-
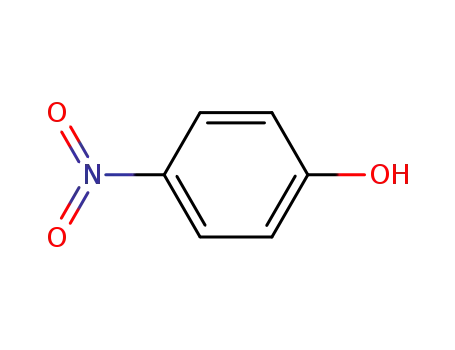
-
100-02-7,78813-13-5,89830-32-0
4-nitro-phenol

-
-
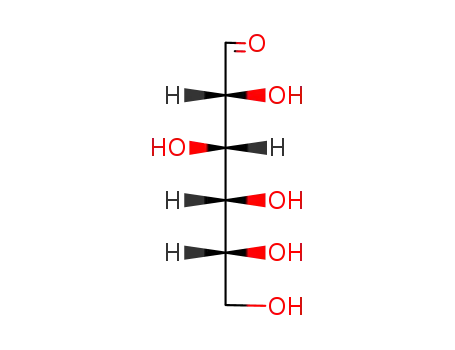
50-99-7
D-glucose
| Conditions | Yield |
|---|---|
|
4-nitrophenyl-β-D-glucoside;
With
C15H27ClCuN3O6(1+)*ClO4(1-); dihydrogen peroxide; triethylamine;
In
water;
at 60 ℃;
for 2h;
Inert atmosphere;
In
water;
Reagent/catalyst;
Catalytic behavior;
Kinetics;
Inert atmosphere;
|
38.3% |
|
With
water;
for 0.333333h;
Rate constant;
acetate buffer pH 5, β-D-glucosidase;
|
|
|
With
β-D-glucopyranosidase;
In
water;
at 40 ℃;
for 1.5h;
Product distribution;
0.1M acetate buffer (pH=4.2), mechanism of action of enzyme;
|
|
|
With
sodium acetate buffer; β-D-glucan exohydrolase isoenzyme ExoI;
at 37 ℃;
Mechanism;
rate of hydrolysis relative to various β-linked oligo-, polysaccharides and aryl β-D-glucosides, other reagents: β-D-glucan exohydrolase isoenzyme ExoII, Na citrate-Na3PO4 buffer, pH 5.25, other pH values, other temperature, various reaction time;
|
|
|
With
sodium phosphate buffer; vanilla bean β-D-glucosidas; Vanilla planifolia Andrews;
at 40 ℃;
pH=7;
Enzyme kinetics;
|
|
|
With
Dalbergia cochinchinensis Pierre dalcochinase N189F mutant; sodium acetate;
In
water;
at 30 ℃;
for 0.0833333h;
pH=5;
Reagent/catalyst;
Kinetics;
Enzymatic reaction;
|
|
|
With
β-glucosidase I from Prunus domestica seeds; citric acid;
at 45 ℃;
pH=5.5;
Temperature;
pH-value;
Time;
Reagent/catalyst;
Kinetics;
aq. phosphate buffer;
Enzymatic reaction;
|
|
|
With
C-terminally His6-tagged Oryza sativa L. β-glucosidase OsTAGG2;
In
aq. acetate buffer;
at 37 ℃;
for 0.166667h;
pH=5;
pH-value;
Temperature;
Reagent/catalyst;
Kinetics;
Catalytic behavior;
Enzymatic reaction;
|
|
|
With
Aspergillus niger ASKU28 glycoside hydrolase family 3 β-glucosidase D487A mutant;
In
aq. buffer;
at 40 ℃;
for 0.166667h;
pH=4.0;
Reagent/catalyst;
Kinetics;
Enzymatic reaction;
|
|
|
With
disodium hydrogenphosphate; Aspergillus niger β-glucosidase; water; citric acid;
at 50 ℃;
for 0.166667h;
pH=4.8;
Reagent/catalyst;
pH-value;
Temperature;
Kinetics;
Enzymatic reaction;
|
|
|
With
recombinant Solanum torvum GH3 β-glucosidase with a polyhistidine tag;
at 37 ℃;
pH=4.5 - 5;
Kinetics;
Enzymatic reaction;
|
|
|
With
rice transglycosidase Os9BGlu31 (wild type); water;
In
aq. acetate buffer;
at 30 ℃;
for 0.5h;
pH=4.5;
Reagent/catalyst;
Kinetics;
Enzymatic reaction;
|
-
-
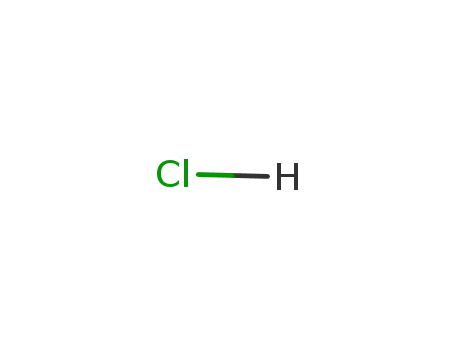
7647-01-0,15364-23-5
hydrogenchloride

-
-
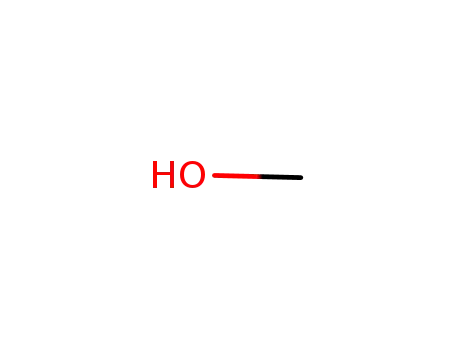
67-56-1
methanol

-
-
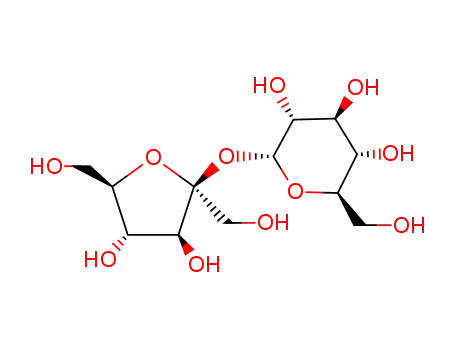
57-50-1
Sucrose

-

-
50-99-7
D-glucose

-
-
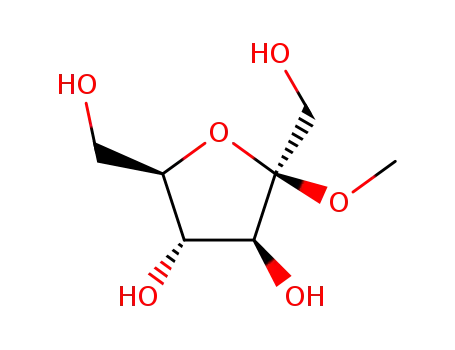
13403-14-0,15219-93-9,16975-88-5,51295-55-7,51295-56-8,52443-09-1,60504-77-0,60504-79-2,63526-47-6,69460-17-9,92283-23-3
methyl β-D-fructofuranoside

-
-
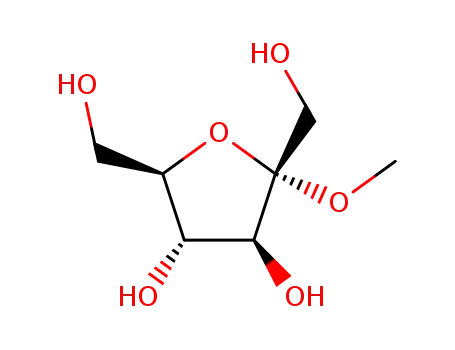
13403-14-0,15219-93-9,16975-88-5,51295-55-7,51295-56-8,52443-09-1,60504-77-0,60504-79-2,63526-47-6,69460-17-9,92283-23-3
methyl α-D-fructofuranoside
| Conditions | Yield |
|---|---|
|
at 20 ℃;
|
50-99-7 Upstream products
-
57-48-7
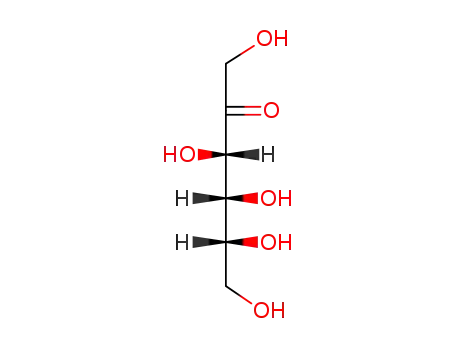
D-Fructose
-
50-99-7
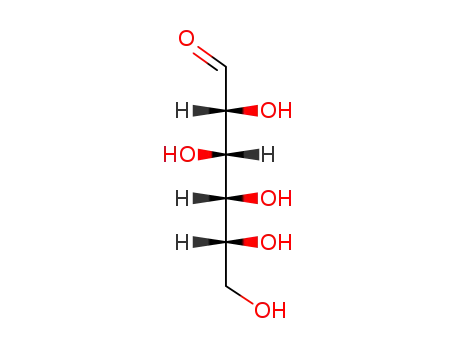
glucose
-
97-30-3
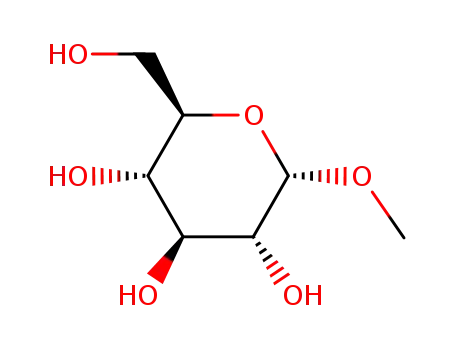
methyl-alpha-D-glucopyranoside
-
90-80-2
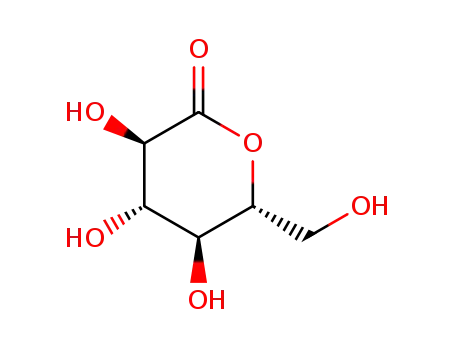
D-Glucono-1,5-lactone
50-99-7 Downstream products
-
143858-30-4
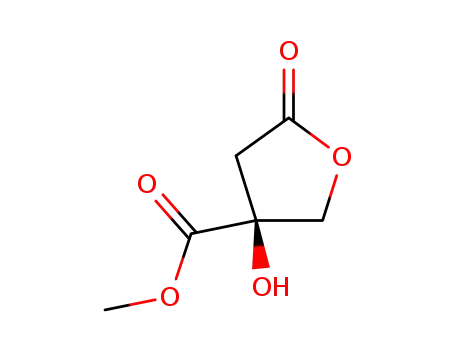
(-)-2-hydroxyparaconic acid methylester
-
7140-75-2
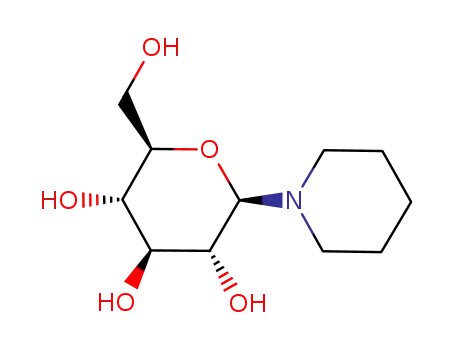
1-(β-D-glucopyranosyl)-piperidine
-
6291-16-3
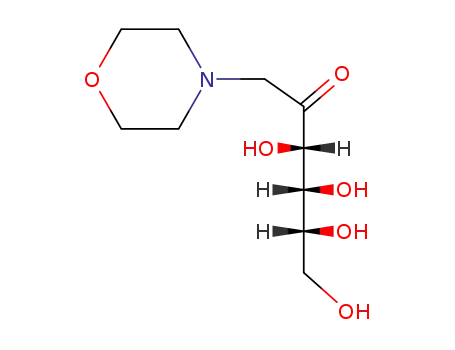
1-deoxy-1-morpholino-D-fructose
-
57-48-7

D-Fructose
Relevant Products
-
Tesamorelin
CAS:218949-48-5
-
Gliclazide
CAS:21187-98-4
-
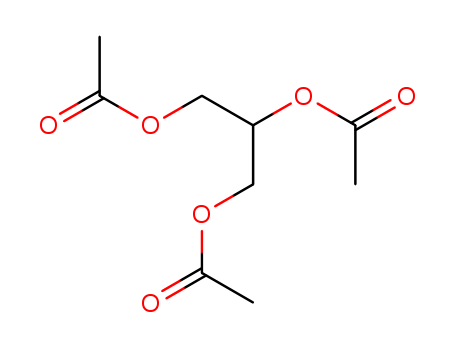
Triacetin
CAS:102-76-1