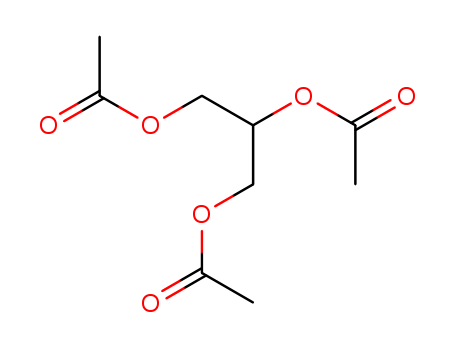
102-76-1
- Product Name:Triacetin
- Molecular Formula:C9H14O6
- Purity:99%
- Molecular Weight:218.207
Product Details;
CasNo: 102-76-1
Molecular Formula: C9H14O6
Appearance: colourless liquid with a bitter taste
Export Top Purity Triacetin 102-76-1 In Stock
- Molecular Formula:C9H14O6
- Molecular Weight:218.207
- Appearance/Colour:colourless liquid with a bitter taste
- Vapor Pressure:0.0141mmHg at 25°C
- Melting Point:3 °C(lit.)
- Refractive Index:n25/D 1.429-1.431(lit.)
- Boiling Point:258 °C at 760 mmHg
- Flash Point:93.9 °C
- PSA:78.90000
- Density:1.16 g/mL at 25 °C(lit.)
- LogP:0.04430
Triacetin(Cas 102-76-1) Usage
|
Production |
It can be derived from the esterification of glycerol and acetic acid. After preheating glycerol to 50-60 ° C, add acetic acid, benzene and sulfuric acid. Heat and stir for refluxing dehydration, and recycle the benzene. Then add acetic anhydride for heating of 4h. After cooling, the mixture was neutralized with 5% sodium carbonate to pH 7, and the crude layer was dried and the crude oil was dried with calcium chloride. Distill under reduced pressure, collect the 128-131 ° C (0.93 kPa) fraction, namely glycerol triacetate. |
|
Content analysis |
Accurately weigh about 1g of the sample, put it into a suitable pressure bottle, add 25 mL of 1mol / L. potassium hydroxide solution and 15 mL of isopropyl alcohol, add stopper, wrap with cloth and put it in a canvas bag. Put it into the water bath of 98 ℃ ± 2 ℃ for 1h, and the water level in the water bath should be slightly higher than the bottle level. Take the bottle out from the bag, cool it to room temperature in the air, unfold the cloth and stopper to release the residual pressure in the bottle, and then remove the cloth. Add 6 to 8 drops of phenolphthalein test solution (TS-167), apply 0.5mol / L sulfuric acid for titration of excess alkali until the pink could just disappeared. At the same time, perform a blank test. Each mL of 0.5mol / L sulfuric acid is equivalent to 36.37 mg of glyceryl triacetate (C9H14O6). |
|
Toxicity |
ADI is not subject to special provisions (FAO / WHO, 2001). GR.AS (FDA, § 182.1901, 2000). LD50 3000mg / kg (rat, oral). |
|
Production Methods |
Triacetin is prepared by the esterification of glycerin with acetic anhydride. |
|
Preparation |
By direct reaction of glycerol with acetic acid in the presence of Twitchell’s reagent, or in benzene solution of glycerol and boiling acetic acid in the presence of a cationic resin (Zeo-Karb H) pretreated with dilute H2SO4. |
|
Manufacturing Process |
200 grams of allyl acetate, 450 grams of glacial acetic acid and 3.71 grams of cobaltous bromide were charged to the reactor and the mixture was heated to 100°C. Pure oxygen was then introduced into the reactor below the surface of the liquid reaction mixture at the rate of 0.5 standard cubic feet per hour. Initially, all of the oxygen was consumed, but after a period of time oxygen introduced into the mixture passed through unchanged. During the course of the reaction, a small quantity of gaseous hydrogen bromide (a total of 1.9 grams) was introduced into the reaction zone, along with the oxygen. The reaction was allowed to continue for 6 hours following which the reaction mixture was distilled. Essentially complete conversion of the allyl acetate took place. A yield of 116 grams of glycerol triacetate was obtained, this being accomplished by distilling the glycerol triacetate overhead from the reaction mixture, at an absolute pressure of approximately 13 mm of mercury. |
|
Therapeutic Function |
Topical antifungal |
|
Pharmaceutical Applications |
Triacetin is mainly used as a hydrophilic plasticizer in both aqueous and solvent-based polymeric coating of capsules, tablets, beads, and granules; typical concentrations used are 10–35% w/w. Triacetin is used in cosmetics, perfumery, and foods as a solvent and as a fixative in the formulation of perfumes and flavors. |
|
Contact allergens |
Triacetin is a component of cigarette filters, which induced a contact dermatitis in a worker at a cigarette manufactory. |
|
Safety Profile |
Poison by ingestion. Moderately toxic by intraperitoneal, subcutaneous, and intravenous routes. An eye irritant. Combustible when exposed to heat, flame, or powerful oxidizers. To fight fire, use alcohol foam, water, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. |
|
Safety |
Triacetin is used in oral pharmaceutical formulations and is generally regarded as a relatively nontoxic and nonirritant material at the levels employed as an excipient. LD50 (dog, IV): 1.5 g/kg LD50 (mouse, IP): 1.4 g/kg LD50 (mouse, IV): 1.6 g/kg LD50 (mouse, oral): 1.1 g/kg LD50 (mouse, SC): 2.3 g/kg LD50 (rabbit, IV): 0.75 g/kg LD50 (rat, IP): 2.1 g/kg LD50 (rat, oral): 3 g/kg LD50 (rat, SC): 2.8 g/kg |
|
storage |
Triacetin is stable and should be stored in a well-closed, nonmetallic container, in a cool, dry place. |
|
Incompatibilities |
Triacetin is incompatible with metals and may react with oxidizing agents. Triacetin may destroy rayon fabric. |
|
Regulatory Status |
GRAS listed. Accepted in Europe as a food additive in certain applications. Included in the FDA Inactive Ingredients Database (oral capsules and tablets and gels). Included in nonparenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients. |
|
Chemical properties |
Colorless, odorless oily liquid. It is miscible with ethanol, ether, benzene, chloroform and other organic solvents, soluble in acetone, insoluble in mineral oil. Slightly soluble in water. 25 ° C in water solubility of 5.9g / 100ml. |
|
Definition |
ChEBI: A triglyceride obtained by acetylation of the three hydroxy groups of glycerol. It has fungistatic properties (based on release of acetic acid) and has been used in the topical treatment of minor dermatophyte infections. |
|
Taste threshold values |
Sweet and creamy with an oily mouthfeel. |
|
General Description |
Triacetin is a triester of glycerin and acetic acid that occurs naturally in papaya. It is mainly used as a synthetic flavoring agent in ice-creams, nonalcoholic beverages and baked goods. |
InChI:InChI=1/C9H14O6/c1-6(10)13-4-9(15-8(3)12)5-14-7(2)11/h9H,4-5H2,1-3H3
102-76-1 Relevant articles
Selective esterification of glycerol to bioadditives over heteropoly tungstate supported on Cs-containing zirconia catalysts
Jagadeeswaraiah,Balaraju,Prasad, P.S. Sai,Lingaiah
, p. 166 - 170 (2010)
Esterification of glycerol with acetic a...
Catalytic hydrogenolysis of glycerol into propyl acetate with ruthenium complexes
Xu, Zichen,Gong, Honghui,Chen, Manyu,Luo, Ruihan,Qian, Wei,Peng, Qingpo,Hou, Zhenshan
, (2019)
Ru complexes have been utilized as catal...
Synthesis of Triacetin and Evaluation on Motor
Lacerda, Claudia V.,Carvalho, Maria J.S.,Ratton, Alice R.,Soares, Itania P.,Borges, Luiz E.P.
, p. 1625 - 1631 (2015)
Triacetin (or glycerol triacetate) was o...
Optimization of N-methyl-N-[tert-butyldimethylsilyl]- trifluoroacetamide as a derivatization agent for determining isotopic enrichment of glycerol in very-low density lipoproteins
Adiels, Martin,Larsson, Thomas,Sutton, Pauline,Taskinen, Marja-Riitta,Boren, Jan,Fielding, Barbara A.
, p. 586 - 592 (2010)
Stable isotope kinetic studies play an i...
An efficient and sustainable production of triacetin from the acetylation of glycerol using magnetic solid acid catalysts under mild conditions
Sun, Jinyan,Tong, Xinli,Yu, Linhao,Wan, Jun
, p. 115 - 122 (2016)
The efficient and selective acetylation ...
Alternative carbon based acid catalyst for selective esterification of glycerol to acetylglycerols
Sánchez, Julián A.,Hernández, Diana L.,Moreno, Jorge A.,Mondragón, Fanor,Fernández, Jhon J.
, p. 55 - 60 (2011)
Carbon-based acid catalysts with porous ...
Synthesis of bio-additives: Acetylation of glycerol over zirconia-based solid acid catalysts
Reddy, Padigapati S.,Sudarsanam, Putla,Raju, Gangadhara,Reddy, Benjaram M.
, p. 1224 - 1228 (2010)
Acetylation of glycerol with acetic acid...
Design of a highly active silver-exchanged phosphotungstic acid catalyst for glycerol esterification with acetic acid
Zhu, Shanhui,Gao, Xiaoqing,Dong, Fang,Zhu, Yulei,Zheng, Hongyan,Li, Yongwang
, p. 155 - 163 (2013)
A series of highly active, selective, an...
SO42-/SnO2: Efficient, chemoselective, and reusable catalyst for acylation of alcohols, phenols, and amines at room temperature
Satam, Jitendra R.,Gawande, Manoj B.,Deshpande, Sameer S.,Jayaram, Radha V.
, p. 3011 - 3020 (2007)
SO42-/SnO2 was employed for the acylatio...
Heteropolyanion-based ionic liquids: Reaction-induced self-separation catalysts for esterification
Leng, Yan,Wang, Jun,Zhu, Dunru,Ren, Xiaoqian,Ge, Hanqing,Shen, Lei
, p. 168 - 171 (2009)
(Figure Presented) It comes out in the w...
Hydrogenation of Carbon Monoxide to Methanol and Ethylene Glycol by Homogeneous Ruthenium Catalysts
Dombek, B. Duane
, p. 6855 - 6857 (1980)
-
Metabolic engineering of: Escherichia coli for production of non-natural acetins from glycerol
Jeong, Seong-Hee,Joo, Seongjoon,Kim, Kyung-Jin,Kim, Seon-Won,Seo, Hogyun,Sohn, Jung-Hoon,Tseten, Tenzin,Wang, Chonglong,Zada, Bakht
, p. 7788 - 7802 (2020)
Mono-, di- and triacetin are three glyce...
Novel double-SO3h functionalized ionic liquid for acetylation
Zhu, Lili,Liang, Xuezheng
, p. 684 - 688 (2012)
Novel double-SO3H functionalized ionic l...
Screening of different solid acid catalysts for glycerol acetylation
Testa, Maria Luisa,La Parola, Valeria,Liotta, Leonarda F.,Venezia, Anna Maria
, p. 69 - 76 (2013)
Different solid acid catalysts, sulfonic...
Continuous synthesis of glycerol acetates in supercritical carbon dioxide using Amberlyst 15
Rezayat, Marzieh,Ghaziaskar, Hassan S.
, p. 710 - 715 (2009)
Continuous esterification of glycerol wi...
Investigation of Sn(IV) catalysts in glycerol acetylation
Altino, Felyppe M. R. S.,Bortoluzzi, Janaína H.,Meneghetti, Simoni M. P.,da Silva, Débora S.
, (2020)
The catalysts dibutyltin dichloride (Bu2...
Combining clays and ultrasound irradiation for an o-acetylation reaction of N-glucopyranosyl and other molecules
De Oliveira, Ronaldo N.,De Xavier, Augusto L.,Guimaraes, Bruna M.,Melo, Valentina N. E.,Valena, Wagner O.,Nascimento Do, Wilson S.,Da Costa, Pollyanna L. F.,Camara, Celso A.
, p. 2610 - 2614 (2014)
A convenient, efficient and fast acetyla...
-
Whalen
, p. 4973 (1978)
-
Co-production of butyrate methyl ester and triacetylglycerol from tributyrin and methyl acetate
Battistel, Ezio,Calaprice, Chiara,Gualdi, Enrico,Rebesco, Elena,Usai, Elisabetta Maria
, p. 149 - 157 (2011)
The simultaneous synthesis of butyric ac...
POSS-derived solid acid catalysts with excellent hydrophobicity for highly efficient transformations of glycerol
Leng, Yan,Zhao, Jiwei,Jiang, Pingping,Lu, Dan
, p. 875 - 881 (2016)
Novel excellent hydrophobic POSS-derived...
Solventless acetylation of alcohols and phenols catalyzed by supported iron oxide nanoparticles
Rajabi, Fatemeh,Luque, Rafael
, p. 129 - 132 (2014)
Supported iron oxide nanoparticles on si...
Acetylation of alcohols and phenols with acetic anhydride under solvent-free conditions using an ionic liquid based on morpholine as a recoverable and reusable catalyst
Yue, Caibo,Liu, Qingqing,Yi, Tingfeng,Chen, Yun
, p. 975 - 978 (2010)
Rapid and efficient acetylation of alcoh...
-
Sokol'skii,Knunjanz
, (1961)
-
-
Sussman
, p. 1230 (1946)
-
A cheap, simple, and versatile method for acetylation of alcohols and phenols and selective deprotection of aromatic acetates under solvent-free condition
Rajabi, Fatemeh,Saidi, Mohammad R.
, p. 483 - 491 (2005)
Acyclic and cyclic acetates of various a...
A heterogeneous cobalt(II) Salen complex as an efficient and reusable catalyst for acetylation of alcohols and phenols
Rajabi, Fatemeh
, p. 395 - 397 (2009)
Acetylation of various alcohols and phen...
Selective Synthesis of Carbonates from Glycerol, CO 2, and Alkyl Halides Using tert -Butyltetramethylguanidine
Mihara, Masatoshi,Moroga, Kaname,Iwasawa, Tetsuo,Nakai, Takeo,Ito, Takatoshi,Ohno, Toshinobu,Mizuno, Takumi
, p. 1759 - 1764 (2018)
Herein, we describe the guanidine-promot...
Zr-modified hierarchical mordenite as heterogeneous catalyst for glycerol esterification
Popova, Margarita,Lazarova, Hristina,Kalvachev, Yuri,Todorova, Totka,Szegedi, ágnes,Shestakova, Pavletta,Mali, Gregor,Dasireddy, Venkata D.B.C.,Likozar, Bla?
, p. 10 - 14 (2017)
Hierarchical mordenite catalysts were pr...
Highly efficient and versatile acetylation of alcohols catalyzed by cerium(III) triflate
Dalpozzo, Renato,De Nino, Antonio,Maiuolo, Loredana,Procopio, Antonio,Nardi, Monica,Bartoli, Giuseppe,Romeo, Roberto
, p. 5621 - 5624 (2003)
Cerium(III) triflate is a powerful catal...
Zeolite catalyzed ring opening of epoxides to acetylated diols with acetic anhydride
Ramesh,Niranjan Reddy,Venugopal,Subrahmanyam,Venkateswarlu
, p. 2599 - 2604 (2001)
HY Zeolite is found to be a versatile ca...
Sulfamic acid catalysed acetylation of alcohols and phenols with acetic anhydride
Jin, Tong-Shou,Ma, Van-Ran,Zhang, Zhan-Hui,Li, Tong-Shuang
, p. 3173 - 3177 (1998)
An easy acetylation of alcohols and phen...
Acetylation of Glycerol over Highly Stable and Active Sulfated Alumina Catalyst: Reaction Mechanism, Kinetic Modeling and Estimation of Kinetic Parameters
Pankajakshan, Arun,Pudi, Satyanarayana Murty,Biswas, Prakash
, p. 98 - 111 (2018)
The Langmuir–Hinshelwood–Hougen–Watson (...
SbCl3 as a highly efficient catalyst for the acetylation of alcohols, phenols, and amines under solvent-free conditions
Bhattacharya, Asish K.,Diallo, Mamadou A.,Ganesh, Krishna N.
, p. 1518 - 1526 (2008)
Antimony trichloride has been found to b...
The Esterification of Carboxylic Acid with Alcohol over Hydrous Zirconium Oxide
Takahashi, Kyoko,Shibagaki, Makoto,Matsushita, Hajime
, p. 2353 - 2361 (1989)
The esterification of carboxylic acids w...
Sulphuric acid-functionalized siliceous zirconia as an efficient and reusable catalyst for the synthesis of glycerol triacetate
Abida, Km,Ali, Amjad
, p. 3627 - 3639 (2020)
In the present study, a sulphated silice...
Yttria-zirconia based Lewis acid: An efficient and chemoselective catalyst for acylation reactions
Kumar,Pandey,Bodas,Dongare
, p. 206 - 209 (2001)
Yttria-zirconia based strong Lewis acid ...
Chemical transformation of embelin through dimerization during preparation of a decoction
Kiuchi, Fumiyuki,Suzuki, Noriko,Fukumoto, Yumiko,Goto, Yoshihisa,Mitsui, Mariko,Tsuda, Yoshisuke
, p. 1225 - 1128 (1998)
Embelin, a major constituent of Embelia ...
Acylated oligosaccharides from Klebsiella K63 capsular polysaccharide: depolymerization by partial hydrolysis and by bacteriophage-borne enzymes.
Dutton,Merrifield
, p. 107 - 128 (1982)
The extracellular polysaccharide from Kl...
Ordered mesoporous zirconium silicates as a catalyst for biofuel precursors synthesis
Bu, Quan,Cai, Jin,Mao, Hanping,Vasudevan, Srinivasan Vinju
, (2021/11/16)
Zirconium incorporated three-dimensional...
Biodiesel Glycerin Valorization into Oxygenated Fuel Additives
Catarino, Mónica,Gomes Fonseca, Frederico,Gomes, Jo?o,Soares Dias, Ana Paula
, (2021/05/26)
Current industrial methods of biodiesel ...
A highly active and stable organic-inorganic combined solid acid for the transesterification of glycerol under mild conditions
Hou, Zhaoyin,Jiang, Yuanyuan,Long, Yihua,Wang, Zhengbao,Ye, Boyong,Zhao, Huaiyuan,Zhou, Ruru
, p. 1772 - 1781 (2021/06/28)
Solid acid catalyst plays a crucial role...
ZWITTERIONIC CATALYSTS FOR (TRANS)ESTERIFICATION: APPLICATION IN FLUOROINDOLE-DERIVATIVES AND BIODIESEL SYNTHESIS
-
Paragraph 0011; 0027, (2021/01/29)
An amide/iminium zwitterion catalyst has...
102-76-1 Process route
-
-
60-01-5
tributyrin

-
-
79-20-9
acetic acid methyl ester

-
-
623-42-7
butanoic acid methyl ester

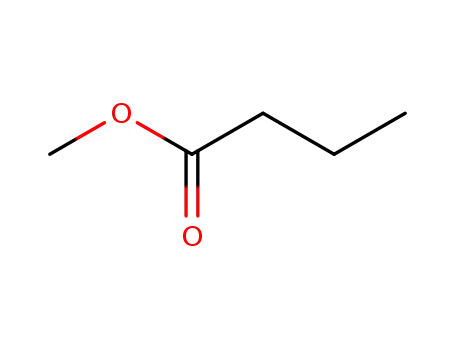
-
-
102-76-1
triacetylglycerol

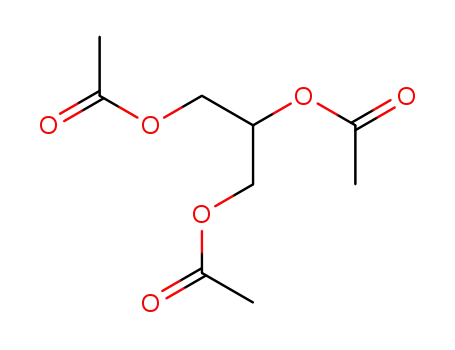
-
-
57416-28-1
3-Butyryl-1,2-diacetyl-sn-glycerin
| Conditions | Yield |
|---|---|
|
With
sodium methylate;
at 60 ℃;
for 0.2h;
|
68 %Chromat. 25 %Chromat. |
-

-
60-01-5
tributyrin

-

-
79-20-9
acetic acid methyl ester

-

-
623-42-7
butanoic acid methyl ester

-

-
102-76-1
triacetylglycerol

-

-
57416-28-1
3-Butyryl-1,2-diacetyl-sn-glycerin

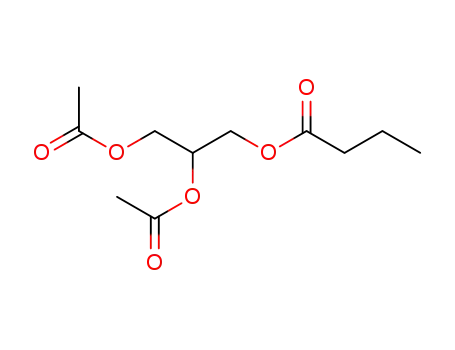
-
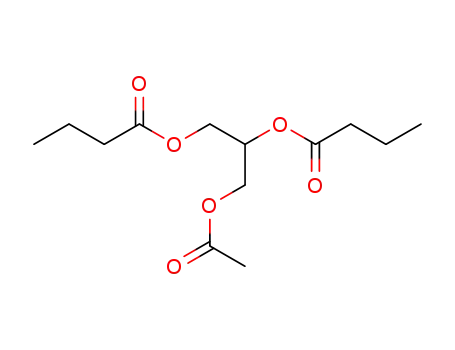
-
65235-08-7
Aceto-dibutyrin
| Conditions | Yield |
|---|---|
|
With
trifluorormethanesulfonic acid; acetic acid;
at 130 ℃;
for 20h;
|
69 %Chromat. 25 %Chromat. 5 %Chromat. |
102-76-1 Upstream products
-
96-11-7
1,2,3-tribromopropane
-
127-08-2
potassium acetate
-
563-63-3
silver(I) acetate
-
108-24-7
acetic anhydride
102-76-1 Downstream products
-
79-20-9

acetic acid methyl ester
-
56-81-5
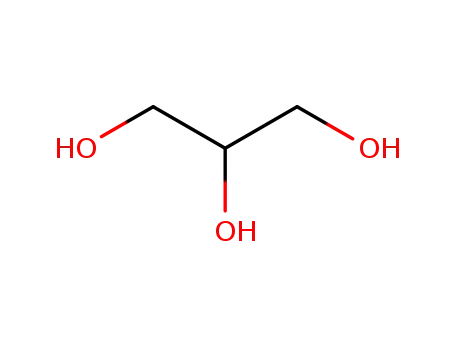
glycerol
-
504-40-5
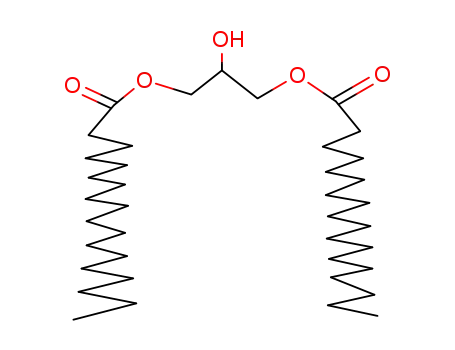
1,3-distearoylglycerol
-
141-78-6
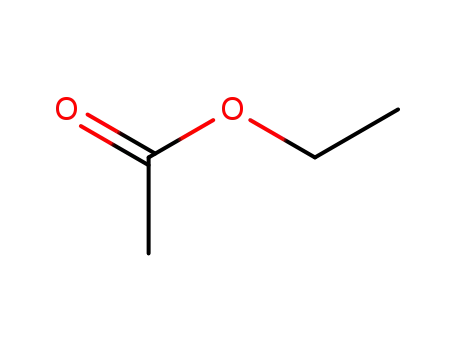
ethyl acetate
Relevant Products
-
Tesamorelin
CAS:218949-48-5
-
D(+)-Glucose
CAS:50-99-7
-
Agar
CAS:9002-18-0