98-80-6
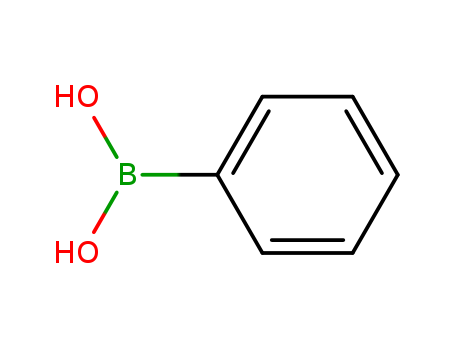
- Product Name:Phenylboronic acid
- Molecular Formula:C6H7BO2
- Purity:99%
- Molecular Weight:121.931
Product Details;
CasNo: 98-80-6
Molecular Formula: C6H7BO2
Appearance: white to light yellow crystal powder
Quality Factory Sells Top Purity 99% Phenylboronic acid 98-80-6 with Safe Delivery
- Molecular Formula:C6H7BO2
- Molecular Weight:121.931
- Appearance/Colour:white to light yellow crystal powder
- Vapor Pressure:0.00446mmHg at 25°C
- Melting Point:216-219 °C(lit.)
- Refractive Index:1.534
- Boiling Point:265.856 °C at 760 mmHg
- PKA:8.83(at 25℃)
- Flash Point:114.586 °C
- PSA:40.46000
- Density:1.139 g/cm3
- LogP:-0.63360
Phenylboronic acid(Cas 98-80-6) Usage
|
Chemical Description |
Phenylboronic acid is an organic compound containing a boronic acid functional group attached to a phenyl group. |
|
Used in Drug Delivery Systems |
Phenylboronic acid-based glucose-sensitive polymer carriers have emerged as promising platforms for diabetic therapy. These bioresponsive delivery systems mimic the physiological insulin secretion model of the pancreas, enabling precise regulation of hypoglycemic drug release to control blood sugar levels in diabetic patients. Phenylboronic acid derivatives exhibit reversible glucose responsiveness, offering stability, long-term storage capabilities, and enhanced drug release control, making them attractive candidates for advanced drug delivery systems aimed at managing diabetes effectively.[1] |
|
Used in Biological Research |
Phenylboronic acid finds widespread use in biological research, particularly in cell labeling and protein analysis applications. Its stable labeling capabilities make it valuable for visualizing and tracking target molecules in living systems, aiding in various research endeavors related to cell biology, molecular biology, and biochemistry.[2] |
|
Used in Drug Synthesis |
Phenylboronic acid serves as a key compound in the organic synthesis of various drugs and pharmaceutical intermediates. It is utilized in the preparation of Pincer nickel(II) complexes and palladium(II) pyridoxal hydrazone metal rings, which function as catalysts in Suzuki-Miyaura cross-coupling reactions. Additionally, phenylboronic acid is employed in the synthesis of N-type polymers for all-polymer solar cells and as a precursor for the production of efficient and selective mTOR kinase inhibitors, showcasing its significance in drug discovery and development.[3] |
|
Used in Environmental Monitoring |
Phenylboronic acid-based fluorescent probes have been developed for selective detection of mercury ions (Hg2+) and methylmercury ions (CH3Hg+) in groundwater and environmental samples. These probes offer high selectivity and sensitivity, enabling rapid and precise detection of trace mercury levels in real-world scenarios. The specific interaction between phenylboronic acid and mercury ions enables the development of cost-effective and efficient fluorescent probes for environmental monitoring applications, facilitating the identification and mitigation of mercury contamination hazards.[4] |
|
Synthesis Reference(s) |
Organic Syntheses, Coll. Vol. 4, p. 68, 1963The Journal of Organic Chemistry, 49, p. 5237, 1984 DOI: 10.1021/jo00200a045 |
|
General Description |
Phenylboronic acid (PBA) is an organoboronic acid. It behaves as a molecular receptor that can attach to compounds containing cis-diol group. Microwave-assisted Suzuki coupling of aryl chlorides with phenylboronic acid in the presence of Pd/C (catalyst) and water (solvent) has been described. Palladium-catalyzed cross-coupling reaction of phenylboronicacid with haloarenes to afford biaryls has been reported. |
|
Safety Profile |
Poison by intravenous andintraperitoneal routes. Moderately toxic by ingestion.Mildly toxic by skin contact. When heated to decomposition it emitsacrid smoke and irritating fumes. |
InChI:InChI=1/C6H7BO2/c8-7(9)6-4-2-1-3-5-6/h1-5,8-9H
98-80-6 Relevant articles
-
Gilman,Moore
, p. 3609 (1958)
-
Catalytic phenylborylation reaction by iridium(0) nanoparticles produced from hydridoiridium carborane
Yinghuai, Zhu,Chenyan, Koh,Ang, Thiam Peng,Emi,Monalisa, Winata,Louis, Loo Kui-Jin,Hosmane, Narayan S.,Maguire, John A.
, p. 5756 - 5761 (2008)
Well-dispersed iridium(0) nanoparticles ...
Novel biscapped and monocapped tris(dioxime) Mn(II) complexes: X-ray crystal structure of the first cationic tris(dioxime) Mn(II) complex [Mn(CDOH)3BPh]OH (CDOH2 = 1,2-cyclohexanedione dioxime)
Hsieh, Wen-Yuan,Liu, Shuang
, p. 5034 - 5043 (2006)
This report describes the synthesis and ...
Evaluation of borinic acids as new, fast hydrogen peroxide–responsive triggers
Gatin-Fraudet, Blaise,Ottenwelter, Roxane,Le Saux, Thomas,Norsikian, Stéphanie,Pucher, Mathilde,Lombès, Thomas,Baron, Aurélie,Durand, Philippe,Doisneau, Gilles,Bourdreux, Yann,Iorga, Bogdan I.,Erard, Marie,Jullien, Ludovic,Guianvarc’h, Dominique,Urban, Dominique,Vauzeilles, Boris
, (2021/12/23)
Hydrogen peroxide (H2O2) is responsible ...
Fourth subgroup metal complex with rigid annular bridging structure and application of fourth subgroup metal complex
-
Paragraph 0058; 0061-0062, (2021/06/23)
The invention belongs to the technical f...
Boronic, diboronic and boric acid esters of 1,8-naphthalenediol-synthesis, structure and formation of boronium salts
Krempner, Clemens,Manankandayalage, Chamila P.,Unruh, Daniel K.
supporting information, p. 4834 - 4842 (2020/04/27)
The 1,8-naphthalenediolate [1,8-O2C10H8]...
Detection of hydrogen peroxide using dioxazaborocanes: elucidation of the sensing mechanism at the molecular level by NMR and XPS measurements
Caron, Thomas,Palmas, Pascal,Frénois, Céline,Méthivier, Christophe,Pasquinet, Eric,Pradier, Claire-Marie,Serein-Spirau, Fran?oise,Hairault, Lionel,Montméat, Pierre
, p. 4114 - 4121 (2020/03/19)
A fluorescent dioxazaborocane was synthe...
98-80-6 Process route
-
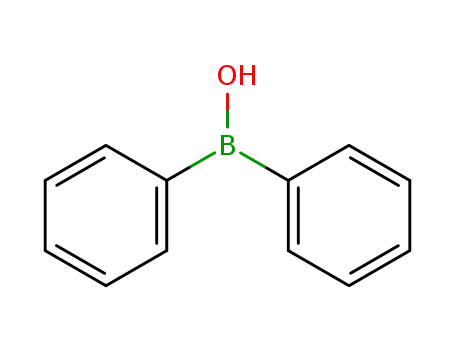
-
2622-89-1
diphenylborinic acid

-
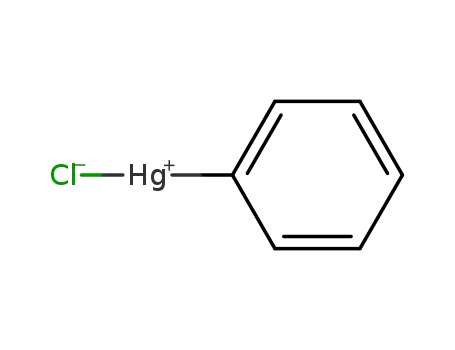
-
100-56-1
phenylmercury(II) chloride

-
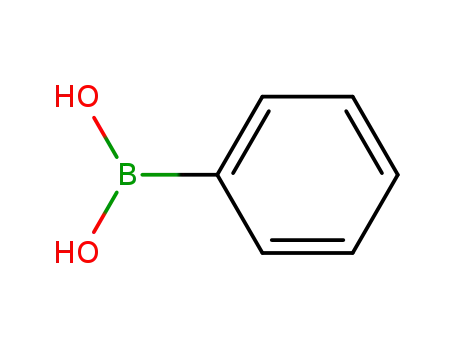
-
98-80-6
phenylboronic acid
| Conditions | Yield |
|---|---|
|
With
mercury dichloride;
|
|
|
With
HgCl2;
|
-
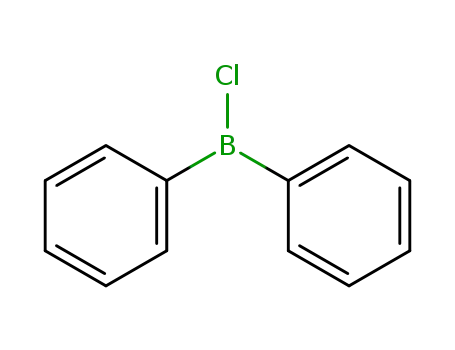
-
3677-81-4
diphenylboronchloride

-

-
100-56-1
phenylmercury(II) chloride

-

-
98-80-6
phenylboronic acid
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: H2O
2: HgCl2
With
H2O; HgCl2;
|
98-80-6 Upstream products
-
60-29-7
diethyl ether
-
688-74-4
boric acid tributyl ester
-
2674-04-6
diphenyl cadmium
-
121-43-7
Trimethyl borate
98-80-6 Downstream products
-
5570-19-4
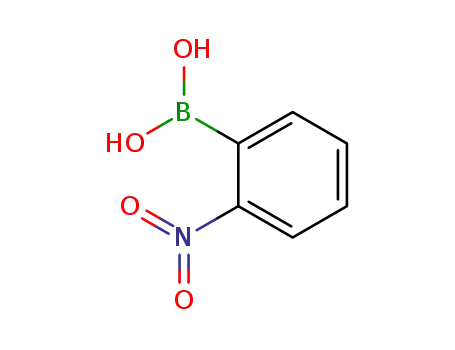
2-nitrophenylboronic acid
-
24067-17-2
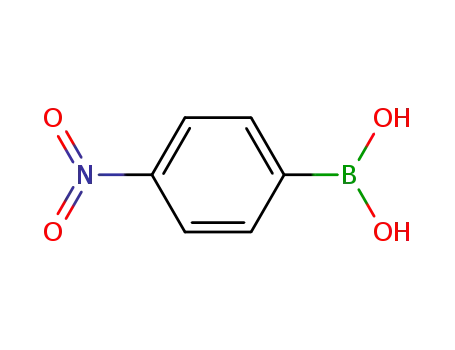
4-nitrophenylboronic acid
-
63181-67-9
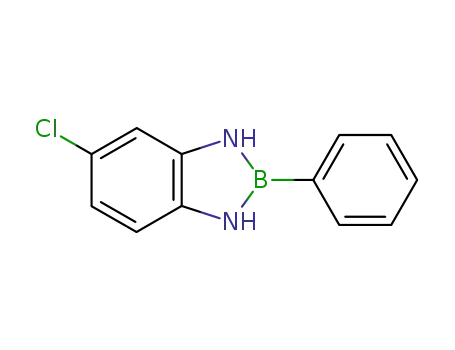
5-chloro-2-phenyl-2,3-dihydro-1H-benzo[1,3,2]diazaborole
-
2479-64-3
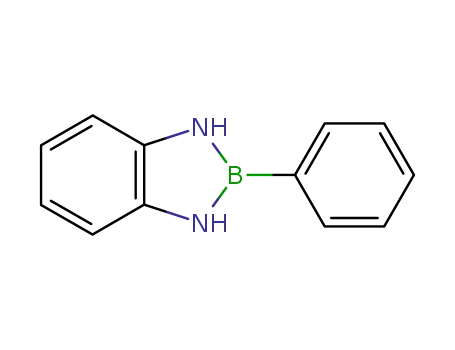
2-phenyl-2,3-dihydro-1H-benzo[1,3,2]diazaborole
Relevant Products
-
5-Bromoindole
CAS:10075-50-0
-
Potassium sulfate(VI)
CAS:7778-80-5
-
Clarithromycin
CAS:81103-11-9