114977-28-5
- Product Name:114977-28-5
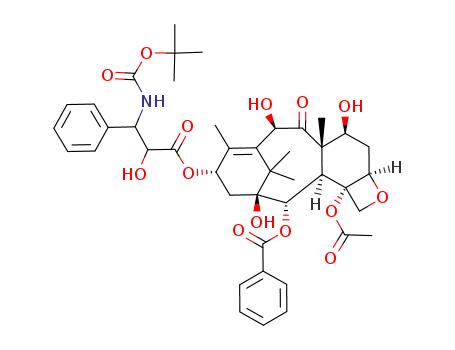
- Molecular Formula:C43H53NO14
- Purity:99%
- Molecular Weight:807.892
Product Details;
CasNo: 114977-28-5
Molecular Formula: C43H53NO14
Appearance: Off-white Cryst
Factory Sells Best Quality 114977-28-5 114977-28-5 with USP
- Molecular Formula:C43H53NO14
- Molecular Weight:807.892
- Appearance/Colour:Off-white Cryst
- Melting Point:232 °C
- Refractive Index:1.618
- Boiling Point:900.5 °C at 760 mmHg
- Flash Point:498.4 °C
- PSA:224.45000
- Density:1.37 g/cm3
- LogP:3.65050
114977-28-5(Cas 114977-28-5) Usage
|
Chemical Properties and Classification |
Docetaxel is a taxane derivative used as a chemotherapeutic drug. Its chemical formula is C43H53NO14, and it belongs to the class of plant alkaloids. It is found crystalline and is commonly prescribed for various malignancies. |
|
Therapeutic Uses |
Docetaxel has been utilized in the treatment of several cancers, including non-small lung cancer, ovarian cancer, triple-negative breast cancer, and metastatic prostate cancer. It acts as a microtubule inhibitor, leading to cell cycle arrest at the G2/M phase and induction of apoptosis. |
|
Challenges in Drug Delivery |
Docetaxel exhibits poor permeability and aqueous solubility, resulting in low oral absorption and bioavailability. It is a substrate for the drug efflux pump P-glycoprotein (P-gp), leading to rapid clearance and drug resistance. |
|
Enhancing Solubility and Delivery |
Common solubility agents like Cremophor EL and Tween80 are employed to enhance the solubility of poorly water-soluble anticancer agents. The development of drug delivery systems is essential to enhance the uptake and accumulation of docetaxel, thereby improving therapeutic efficacy at lower doses. |
|
Metabolism and Transport |
Docetaxel is susceptible to metabolism by enzymes belonging to the cytochrome P450 family. It interacts with transport proteins, with P-glycoprotein being responsible for the majority of its transportation across cellular membranes. |
|
Recent Developments |
The addition of darolutamide, an androgen receptor signaling inhibitor, to therapy with docetaxel has been approved for treating metastatic prostate cancer. Clinical trials are ongoing to investigate the efficacy of darolutamide combined with androgen deprivation therapy (ADT) and docetaxel or cabazitaxel in metastatic castration-resistant prostate cancer patients. |
|
General Description |
The chemical with the CAS number 114977-28-5 is a synthetic organic compound with a molecular formula that has not been disclosed. This chemical is known to be used in the production of pharmaceuticals, agrochemicals, and other specialty chemical products. It is categorized as a potential hazardous substance and is subject to strict regulations regarding its handling, storage, and transportation. Due to its proprietary nature, specific information about its properties and uses is not publicly available. However, it is important to handle this chemical with care and to follow all recommended safety protocols when working with it. |
InChI:InChI=1/C43H53NO14/c1-22-26(55-37(51)32(48)30(24-15-11-9-12-16-24)44-38(52)58-39(3,4)5)20-43(53)35(56-36(50)25-17-13-10-14-18-25)33-41(8,34(49)31(47)29(22)40(43,6)7)27(46)19-28-42(33,21-54-28)57-23(2)45/h9-18,26-28,30-33,35,46-48,53H,19-21H2,1-8H3,(H,44,52)/t26-,27-,28+,30-,31+,32+,33-,35-,41-,42-,43+/m0/s1
114977-28-5 Relevant articles
Semisynthesis method for docetaxel
-
Paragraph 0028; 0033-0035; 0040-0042; 0047-0048, (2020/06/02)
The invention relates to a semisynthesis...
Synthesis of Taxol and Docetaxel by Using 10-Deacetyl-7-xylosyltaxanes
Xue, Baoyu,Zhao, Junhong,Fan, Yange,Chen, Shipeng,Li, Wenfeng,Chen, Jin,Li, Zheng,Wang, Hongxing,Kong, Hongjun
, (2020/02/05)
A mixture of taxols was prepared from 10...
Method for synthesizing cabazitaxel from 10-deacetylbaccatin III
-
, (2019/09/17)
The invention discloses a method for syn...
Method for purifying docetaxel
-
Paragraph 0051-0053, (2019/07/04)
The invention discloses a method for pur...
114977-28-5 Process route
-
-
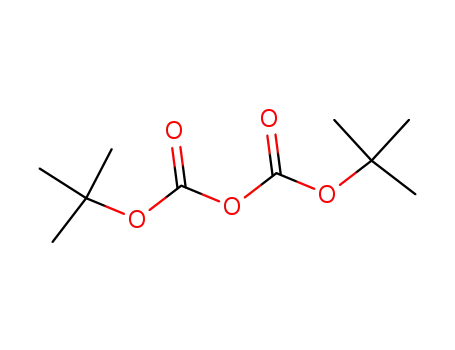
24424-99-5
di-tert-butyl dicarbonate

-
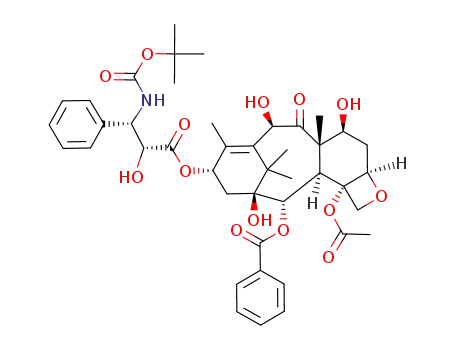
![(2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-acetoxy-9-(((2R,3S)-3-amino-2-hydroxy-3-phenylpropanoyl)oxy)-4,6,11-trihydroxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxet-12-yl benzoate](/upload/2025/4/faf7e6d3-198f-4153-93c4-625c1d26395e.png)
-
133524-69-3,133577-36-3,133577-37-4
(2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-acetoxy-9-(((2R,3S)-3-amino-2-hydroxy-3-phenylpropanoyl)oxy)-4,6,11-trihydroxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxet-12-yl benzoate

-
-
114915-20-7,114977-28-5,114977-29-6,133577-32-9,133577-33-0
Docetaxel
| Conditions | Yield |
|---|---|
|
|
100% |
|
With
acetic acid;
In
ethanol; dichloromethane;
at 25 ℃;
for 3h;
Product distribution / selectivity;
|
89% |
|
In
methanol;
at 25 ℃;
for 15h;
|
70 % Chromat. |
|
In
ethyl acetate;
at 20 ℃;
for 12h;
|
|
|
In
ethanol; dichloromethane;
at 20 ℃;
for 16h;
Product distribution / selectivity;
|
|
|
With
triethylamine;
In
tetrahydrofuran;
at 20 ℃;
|
|
|
In
water; ethyl acetate;
for 1h;
|
|
|
With
dmap;
In
tetrahydrofuran;
at 20 ℃;
Product distribution / selectivity;
|
|
|
With
sodium hydrogencarbonate;
In
water; ethyl acetate;
at 25 - 30 ℃;
pH=7.5 - 8.5;
|
22 g |
-
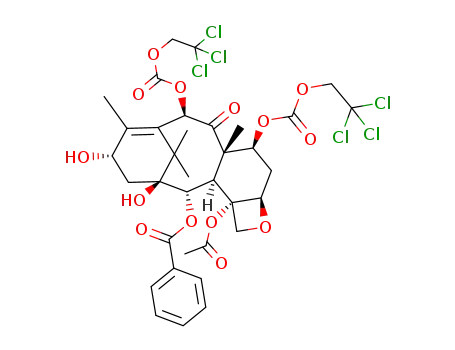
-
95603-44-4
7,10-di-(2,2,2-trichloroethyloxycarbonyl)-10-deacetylbaccatin III

-
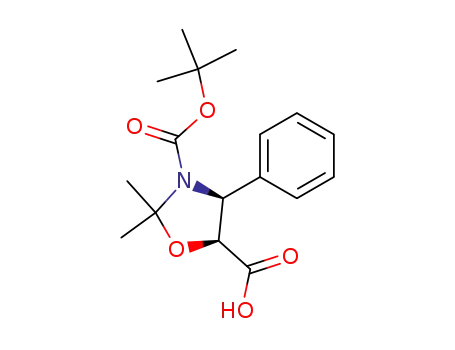
-
(4S,5R)-N-(tert-butyloxycarbonyl)-2,2-dimethyl-4-phenyl-5-oxazolidinecarboxylic acid

-

-
114915-20-7,114977-28-5,114977-29-6,133577-32-9,133577-33-0
Docetaxel
| Conditions | Yield |
|---|---|
|
With
dmap; dicyclohexyl-carbodiimide;
Multistep reaction;
1.) toluene;
|
114977-28-5 Upstream products
-
24424-99-5

di-tert-butyl dicarbonate
-
133524-69-3
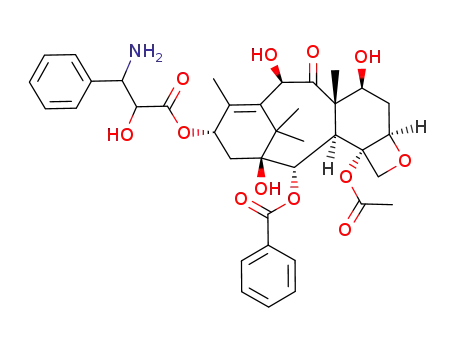
C38H45NO12
-
95603-45-5
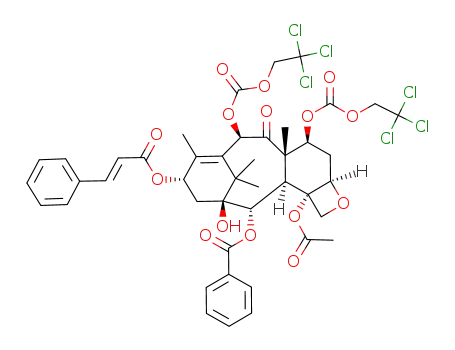
7,10-di(2,2,2-trichloroethyloxy-carbonyl)-13-cinnamoyl-10-deacetyl baccatin III
-
133524-69-3
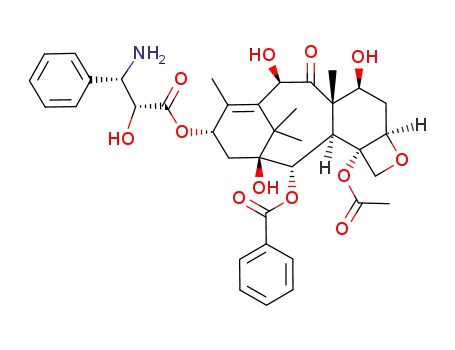
(2aR,4S,4aS,6R,9S,11S,12S,12aR,12bS)-12b-acetoxy-9-(((2R,3S)-3-amino-2-hydroxy-3-phenylpropanoyl)oxy)-4,6,11-trihydroxy-4a,8,13,13-tetramethyl-5-oxo-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-1H-7,11-methanocyclodeca[3,4]benzo[1,2-b]oxet-12-yl benzoate
114977-28-5 Downstream products
-
342613-14-3
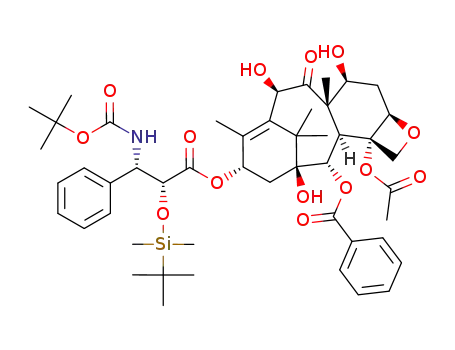
2'-O-(tert-butyldimethylsilyl)docetaxel
-
960155-98-0
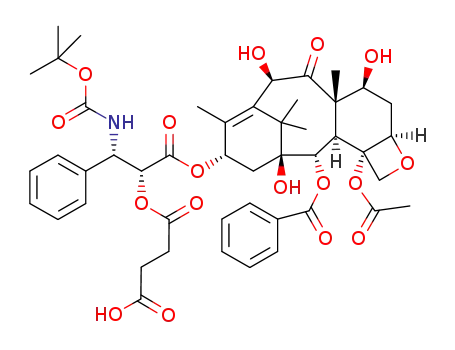
docetaxel 2'-succinic ester
Relevant Products
-
Tesamorelin
CAS:218949-48-5
-
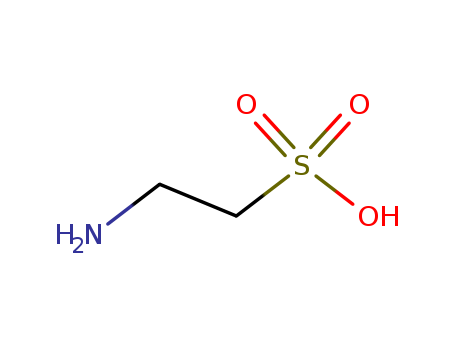
Taurine
CAS:107-35-7
-
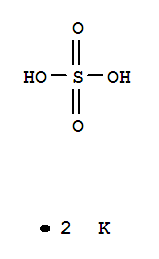
Potassium sulfate(VI)
CAS:7778-80-5