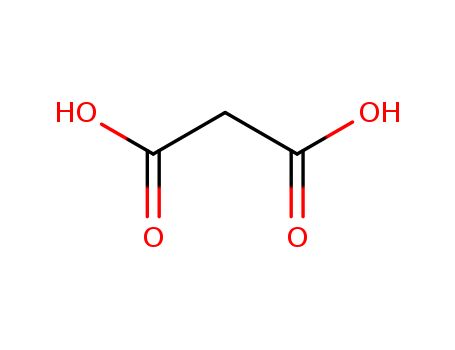
141-82-2
- Product Name:Malonic acid
- Molecular Formula:C3H4O4
- Purity:99%
- Molecular Weight:104.062
Product Details;
CasNo: 141-82-2
Molecular Formula: C3H4O4
Appearance: Crystalline
99% Purity Commercial production Malonic acid 141-82-2 with Cheapest Price
- Molecular Formula:C3H4O4
- Molecular Weight:104.062
- Appearance/Colour:Crystalline
- Vapor Pressure:4.66E-07mmHg at 25°C
- Melting Point:132-135 °C (dec.)(lit.)
- Boiling Point:386.808 °C at 760 mmHg
- Flash Point:201.905 °C
- PSA:74.60000
- Density:1.546 g/cm3
- LogP:-0.45430
Malonic acid(Cas 141-82-2) Usage
|
Chemical Description |
Malonic acid is a dicarboxylic acid with the chemical formula C3H4O4. |
|
Production Methods |
Malonic acid can be produced from fossil resources via petrochemical processes or through microbial fermentation using renewable feedstocks. However, there is growing interest in its biological production via microbial fermentation using renewable feedstocks. |
InChI:InChI=1/C3H4O4/c4-2(5)1-3(6)7/h1H2,(H,4,5)(H,6,7)/p-2
141-82-2 Relevant articles
Investigation of Radical Reactions Important in the Gyoergyi-Turanyi-Field Model of the Belousov-Zhabotinskii Reaction
Foersterling, Horst-Dieter,Stuk, Linda
, p. 7320 - 7325 (1991)
In the Gyoergyi-Turanyi-Field (GTF) mode...
MALONATED ANTHOCYANINS IN MALVACEAE: MALONYLMALVIN FROM MALVA SYLVESTRIS
Takeda, Kosaku,Enoki, Shigeki,Harborne, Jeffrey B.,Eagles, John
, p. 499 - 500 (1989)
A new anthocyanin, malvidin 3-(6"-malony...
An unusual acylated malvidin 3-glucoside from flowers of Impatiens textori Miq. (Balsaminaceae)
Tatsuzawa, Fumi,Saito, Norio,Mikanagi, Yuki,Shinoda, Koichi,Toki, Kenjiro,Shigihara, Atsushi,Honda, Toshio
, p. 672 - 674 (2009)
Acylated malvidin 3-glucoside was isolat...
-
Klemenc, A.,Wechsberg, R.,Wagner, G.
, (1935)
-
Exploring the Promiscuous Enzymatic Activation of Unnatural Polyketide Extender Units in Vitro and in Vivo for Monensin Biosynthesis
Grote, Marius,Schulz, Frank
, p. 1183 - 1189 (2019)
The incorporation of new-to-nature exten...
Diverse structural assemblies of a series of ninhydrin derivatives: Quantitative analyses from experimental and theoretical studies
Hundal, Geeta,Kapoor, Kamal K.,Mahajan, Sheena,Saini, Yeshwinder,Seth, Saikat Kumar
, (2021)
Three ninhydrin derivatives (2–4) have b...
-
Shoppee,C.W.,Hughes,N.W.
, p. 3673 - 3679 (1971)
-
Kinetic modeling of malonylgenistin and malonyldaidzin conversions under alkaline conditions and elevated temperatures
Vaidya, Nirupama A.,Mathias, Kevin,Ismail, Baraem,Hayes, Kirby D.,Corvalan, Carlos M.
, p. 3408 - 3413 (2007)
The conversion and degradation of malony...
Enols derived from malonic acids as intermediates in nitrosation and halogenation
Williams,Graham
, p. 7973 - 7978 (1992)
Malonic acid, methylmalonic acid, ethylm...
Design of bisquinolinyl malonamides as Zn2+ ion-selective fluoroionophores based on the substituent effect
Moriuchi-Kawakami, Takayo,Kawata, Keita,Nakamura, Sho,Koyama, Yoshiaki,Shibutani, Yasuhiko
, p. 9805 - 9813 (2014)
A series of malonamides possessing two q...
Photo-Induced Disproportionation of Iodomalonic Acid
Rabai, Gyula,Hanazaki, Ichiro
, p. 431 - 442 (1995)
The stoichiometry, equilibrium, and kine...
Experimental and mechanistic investigation of an iodomalonic acid-based Briggs-Rauscher oscillator and its perturbations by resorcinol
Cervellati, Rinaldo,Greco, Emanuela,Furrow, Stanley D.
, p. 12888 - 12892 (2010)
Classic Briggs-Rauscher oscillators use ...
Degradation of 2,5-dihydroxy-1,4-benzoquinone by hydrogen peroxide under moderately alkaline conditions resembling pulp bleaching: A combined kinetic and computational study
Hosoya, Takashi,Rosenau, Thomas
, p. 11194 - 11203 (2013)
2,5-Dihydroxy-1,4-benzoquinone (DHBQ) is...
PRUNASIN-6'-MALONATE, A CYANOGENIC GLUCOSIDE FROM MERREMIA DISSECTA
Nahrstedt, Adolf,Jensen, Pia Skjottgaard,Wray, Victor
, p. 623 - 624 (1989)
The cyanogenic glucosides prunasin and 6...
Single particle analysis of secondary organic aerosols formed from 1,4-cyclohexadiene ozonolysis using a laser-ionization single-particle aerosol mass spectrometer
Narukawa, Masahiro,Matsumi, Yutaka,Matsumoto, Jun,Takahashi, Kenshi,Yabushita, Akihiro,Sato, Kei,Imamura, Takashi
, p. 120 - 126 (2008)
Real-time analysis of secondary organic ...
Doxycycline degradation by the oxidative Fenton process
Borghi, Alexandre A.,Silva, Milena F.,Al Arni, Saleh,Converti, Attilio,Palma, Mauri S. A.
, (2015)
Doxycycline is a broad-spectrum tetracyc...
Ozonolysis of α-angelica lactone: a renewable route to malonates
Dell’Acqua, Andrea,Stadler, Bernhard M.,Tin, Sergey,Wille, Lukas,de Vries, Johannes G.
supporting information, p. 10524 - 10527 (2021/10/19)
Industrially relevant intermediates such...
Electrochemical oxidation of diclofenac on CNT and M/CNT modified electrodes
Ferreira, M.,Figueiredo, J. L.,Fonseca, A. M.,Güney, S.,Ku?niarska-Biernacka, I.,Neves, I. C.,Parpot, P.,Pereira, M. F. R.,Soares, O. S. G. P.
, p. 12622 - 12633 (2021/07/25)
The electrochemical oxidation of diclofe...
Hydrolysis of amides to carboxylic acids catalyzed by Nb2O5
Siddiki,Rashed, Md. Nurnobi,Touchy, Abeda Sultana,Jamil, Md. A. R.,Jing, Yuan,Toyao, Takashi,Maeno, Zen,Shimizu, Ken-Ichi
, p. 1949 - 1960 (2021/03/26)
Hydrolysis of amides to carboxylic acids...
141-82-2 Process route
-
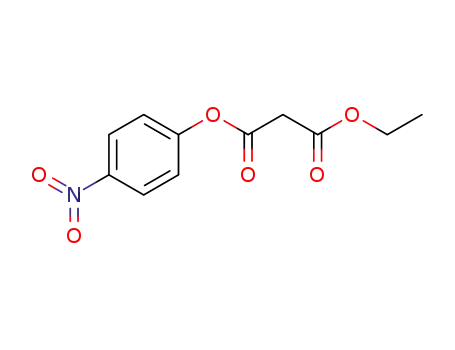
-
24161-55-5
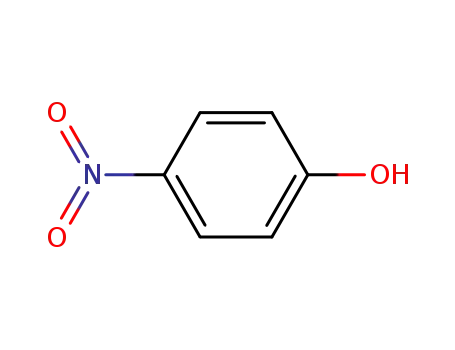
ethyl 4-nitrophenyl malonate

-
-
100-02-7,78813-13-5,89830-32-0
4-nitro-phenol

-
-
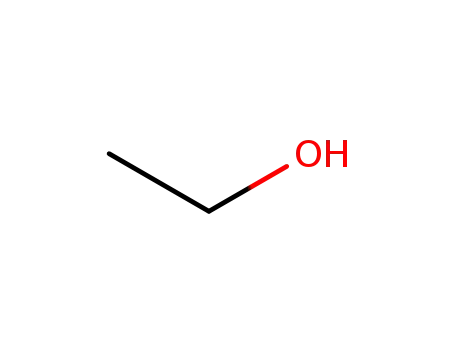
64-17-5
ethanol

-
-
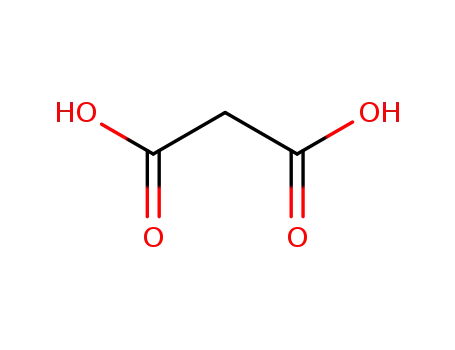
141-82-2
malonic acid
| Conditions | Yield |
|---|---|
|
With
pH = 5.05;
In
water;
at 30.6 ℃;
under 750.06 Torr;
Mechanism;
Rate constant;
Thermodynamic data;
pressure-dependence of rates of elimination; activation parameters for hydrolysis: ΔV(excit.), ΔS(excit.), EA(excit.); var. press.;
|
-
-
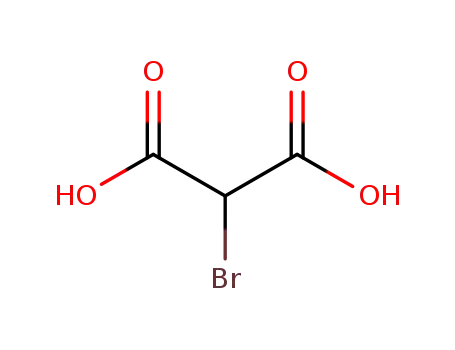
600-31-7
2-bromomalonic acid

-
-
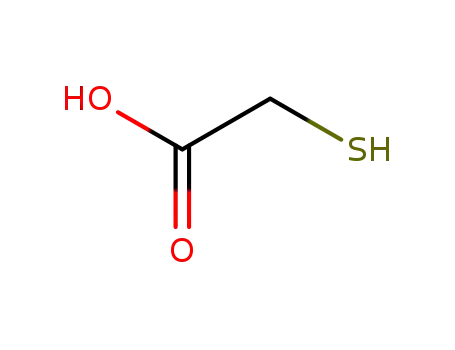
68-11-1
mercaptoacetic acid

-

-
141-82-2
malonic acid

-
-
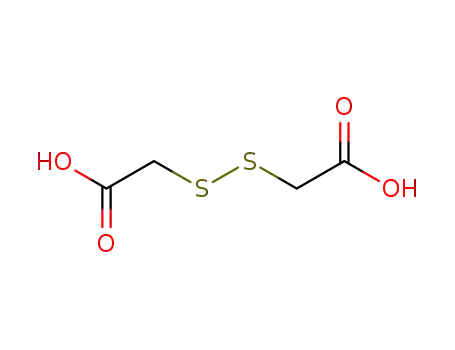
505-73-7
disulfanediyldiacetic acid

-
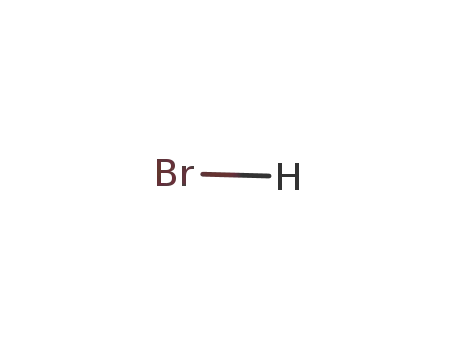
-
10035-10-6,12258-64-9
hydrogen bromide
| Conditions | Yield |
|---|---|
|
Kinetics;
Kinetik der Reaktionen in Wasser;
|
|
|
at 20 - 25 ℃;
|
141-82-2 Upstream products
-
67-52-7
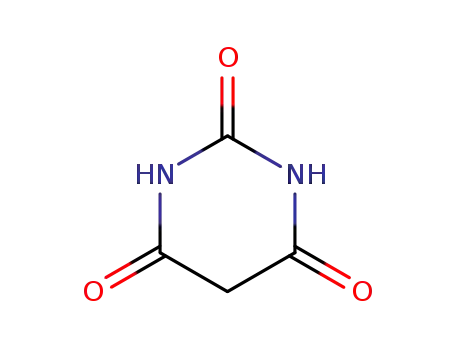
BARBITURIC ACID
-
21577-50-4
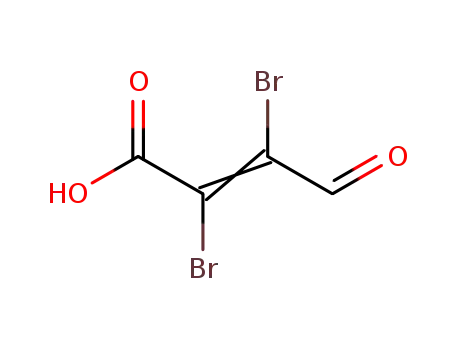
mucobromic acid
-
187737-37-7
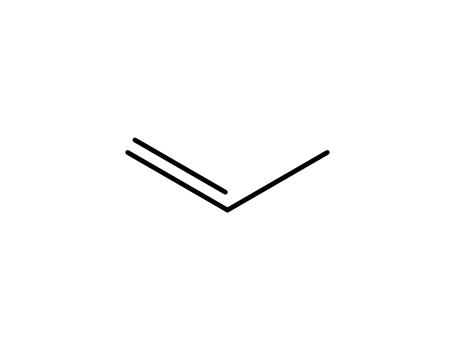
propene
-
504-64-3
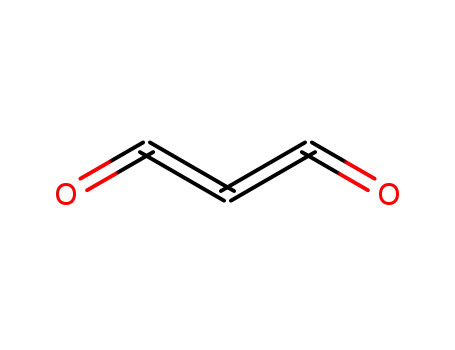
carbon suboxide
141-82-2 Downstream products
-
676551-01-2
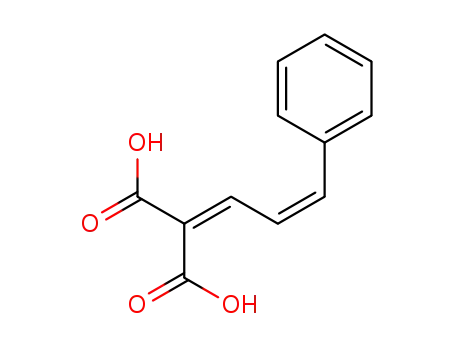
2-((Z)-3-Phenyl-allylidene)-malonic acid
-
1552-94-9
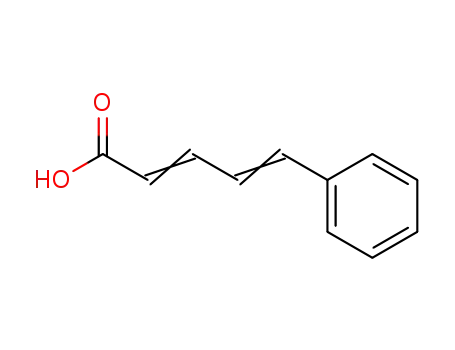
5-phenyl-2,4-pentadienoic acid
-
38489-70-2
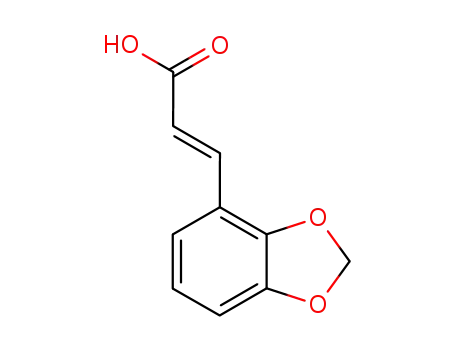
2,3-(methylenedioxy)cinnamic acid
-
110795-27-2
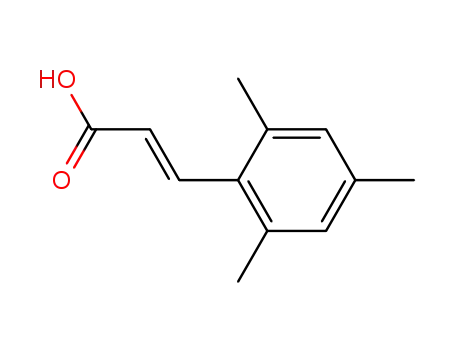
(2E)-3-(2,4,6-trimethylphenyl)propenoic acid
Relevant Products
-
Tesamorelin
CAS:218949-48-5
-
Cisplatin
CAS:15663-27-1
-
Ammonium bromide
CAS:12124-97-9