372-75-8
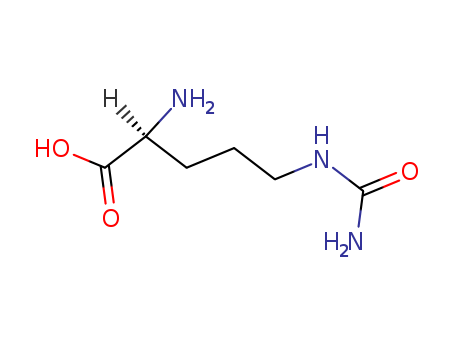
- Product Name:L(+)-Citrulline
- Molecular Formula:C6H13N3O3
- Purity:99%
- Molecular Weight:175.188
Product Details;
CasNo: 372-75-8
Molecular Formula: C6H13N3O3
Appearance: white powder
Offer Chemical Raw Material L(+)-Citrulline 372-75-8 In Stock
- Molecular Formula:C6H13N3O3
- Molecular Weight:175.188
- Appearance/Colour:white powder
- Vapor Pressure:4.7E-07mmHg at 25°C
- Melting Point:214 °C
- Refractive Index:26 ° (C=8, 6mol/L HCl)
- Boiling Point:386.7 °C at 760 mmHg
- PKA:2.43(at 25℃)
- Flash Point:187.7 °C
- PSA:118.44000
- Density:1.289 g/cm3
- LogP:0.63830
L(+)-Citrulline(Cas 372-75-8) Usage
|
Manufacturing Process |
Citrulline is obtained as a result of a reaction of L-arginine hydrochloride with sodium hydroxide, copper oxide and hydrogen sulfide. In practice it is usually used as malate salt |
|
Therapeutic Function |
Stimulant, Detoxicant |
|
Biochem/physiol Actions |
L-Citrulline, when orally administered is found to ameliorate the condition of sickle cell disease in humans. L-citrulline can be a replacement for L-arginine administration in case of defective NO synthetase. L-Citrulline supplementation is found to fulfill the purpose of NO-dependent signaling. |
|
Purification Methods |
Likely impurities are arginine and ornithine. Crystallise S-citrulline from water by adding 5 volumes of EtOH. Also crystallise it from water by addition of MeOH. [Ellenbogen J Am Chem Soc 74 5198 1952, Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 pp 2491-2494 1961, Beilstein 4 IV 2647.] |
|
Bioavailability and Properties |
L-citrulline is a natural precursor of L-arginine and is more bioavailable than L-arginine due to its ability to avoid hepatic first-pass metabolism and longer circulation time. It possesses antioxidant and vasodilation properties and is an integral part of the human nitric oxide system. |
|
Enzymatic Regulation |
The metabolism of L-citrulline involves key enzymes such as nitric oxide synthase (NOS), ornithine transcarbamylase (OTC), and argininosuccinate synthase (ASS), which are responsible for CIT synthesis and catabolism in mammals, respectively. These enzymes regulate CIT's involvement in various metabolic pathways. |
|
Synthesis and Sources |
Citrulline is synthesized from ornithine and carbamoyl phosphate in the urea cycle and is also produced from arginine as a by-product of the reaction catalyzed by NOS family enzymes. It is found primarily in watermelon, cucumbers, and other melons, with watermelon being the most naturally rich source. |
|
Supplementation and Effects |
L-citrulline is commonly supplemented in the form of citrulline malate, and its benefits may be attributed to the synergistic combination of L-citrulline and malate at the intramuscular metabolic level. Pharmacokinetic studies have shown that plasma citrulline reaches peak concentration between 40 and 60 minutes after ingestion, while plasma arginine peaks between 80 and 120 minutes, with elevated concentrations lasting up to 8 hours. |
|
Metabolic Pathways |
L-citrulline plays a crucial role in the urea cycle in the liver, responsible for ammonia elimination in the form of urea. During high-intensity exercise, citrulline supplementation may aid in ammonia detoxification, decrease lactate production, and enhance aerobic utilization of pyruvate, thereby improving muscle function and reducing fatigue. |
|
Metabolic Characteristics |
L-citrulline is an organic compound and a non-essential amino acid synthesized endogenously by the body. It is not involved in protein synthesis but is an important component of metabolic pathways such as arginine biosynthesis, the Arg-CIT-nitric oxide cycle, and the urea cycle. |
|
Role in Sustainable Practices |
Watermelon and its by-products, such as the rind, can be utilized for citrulline extraction, promoting sustainability in the fresh-cut watermelon industry and supporting a circular economy model. |
InChI:InChI=1/C6H13N3O3/c7-4(5(10)11)2-1-3-9-6(8)12/h4H,1-3,7H2,(H,10,11)(H3,8,9,12)/t4-/m0/s1
372-75-8 Relevant articles
Dissecting structural and electronic effects in inducible nitric oxide synthase
Hannibal, Luciana,Page, Richard C.,Haque, Mohammad Mahfuzul,Bolisetty, Karthik,Yu, Zhihao,Misra, Saurav,Stuehr, Dennis J.
, p. 153 - 165 (2015)
Nitric oxide synthases (NOSs) are haem-t...
Mesohaem substitution reveals how haem electronic properties can influence the kinetic and catalytic parameters of neuronal NO synthase
Tejero, Jesus,Biswas, Ashis,Haque, Mohammad Mahfuzul,Wang, Zhi-Qiang,Hemann, Craig,Varnado, Cornelius L.,Novince, Zachary,Hille, Russ,Goodwin, Douglas C.,Stuehr, Dennis J.
, p. 163 - 174 (2011)
NOSs (NO synthases, EC 1.14.13.39) are h...
Neuronal nitric oxide synthase isoforms α and μ are closely related calpain-sensitive proteins
Laine, Romuald,Ortiz De Montellano, Paul R.
, p. 305 - 312 (1998)
The neuronal nitric oxide synthase isofo...
Arginine Deiminase Uses an Active-Site Cysteine in Nucleophilic Catalysis of L-Arginine Hydrolysis
Lu, Xuefeng,Galkin, Andrey,Herzberg, Osnat,Dunaway-Mariano, Debra
, p. 5374 - 5375 (2004)
Arginine deiminase (EC 3.5.3.6) catalyze...
Directed evolution of an antitumor drug (Arginine Deiminase PpADI) for Increased Activity at Physiological pH
Zhu, Leilei,Tee, Kang Lan,Roccatano, Danilo,Sonmez, Burcu,Ni, Ye,Sun, Zhi-Hao,Schwaneberg, Ulrich
, p. 691 - 697 (2010)
Arginine deiminase (ADI; EC 3.5.3.6) has...
L337H mutant of rat neuronal nitric oxide synthase resembles human neuronal nitric oxide synthase toward inhibitors
Fang, Jianguo,Ji, Haitao,Lawton, Graham R.,Xue, Fengtian,Roman, Linda J.,Silverman, Richard B.
, p. 4533 - 4537 (2009)
A common dichotomy exists in inhibitor d...
Mechanisms of catalysis and inhibition operative in the arginine deiminase from the human pathogen Giardia lamblia
Li, Zhimin,Kulakova, Liudmila,Li, Ling,Galkin, Andrey,Zhao, Zhiming,Nash, Theodore E.,Mariano, Patrick S.,Herzberg, Osnat,Dunaway-Mariano, Debra
, p. 149 - 161 (2009)
Giardia lamblia arginine deiminase (GlAD...
Ruthenium(III) readily abstracts NO from L-arginine, the physiological precursor to NO, in the presence of H2O2. A remarkably simple model system for NO synthases.
Marmion,Murphy,Nolan
, p. 1870 - 1871 (2001)
Reaction of [Ru(Hedta)Cl]- with H2O2 in ...
IMPROVED METHOD OF SYNTHESIS AND PURIFICATION OF CITRULLINE
-
Paragraph 00033-00034; 00037-00038, (2020/12/29)
This invention provides for synthesis of...
l-Arginine and nitric oxide synthesis in the cells with inducible NO synthase
Kuropteva,Baider,Nagler,Bogatyrenko,Belaia
, p. 174 - 180 (2019/04/25)
The effect of citrulline and ammonium ch...
Enzyme-immobilized metal-organic framework nanosheets as tandem catalysts for the generation of nitric oxide
Gao, Feng,Lei, Jianping,Ling, Pinghua,Qian, Caihua
supporting information, p. 11176 - 11179 (2020/04/23)
An enzyme-immobilized metal-organic fram...
Method for preparing L-citrulline crude product and L-citrulline prepared through method
-
Paragraph 0033-0108, (2017/05/05)
The invention discloses a method for pre...
372-75-8 Process route
-
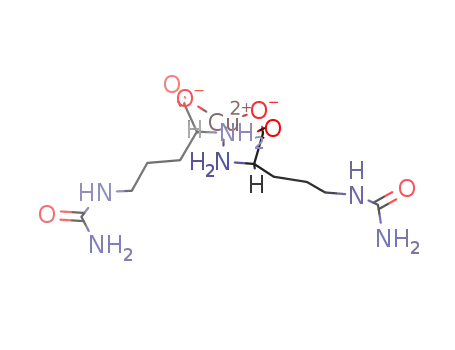
-
L-citrulline copper complex

-
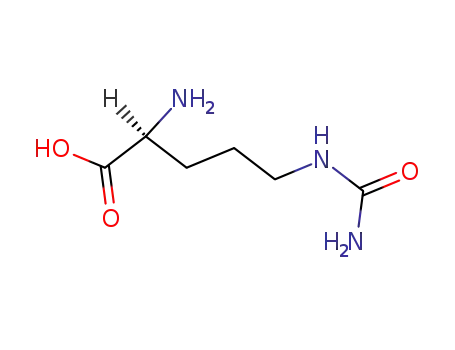
-
372-75-8
Citrulline
| Conditions | Yield |
|---|---|
|
With
sodium sulfide;
In
water;
Reagent/catalyst;
Large scale;
|
-
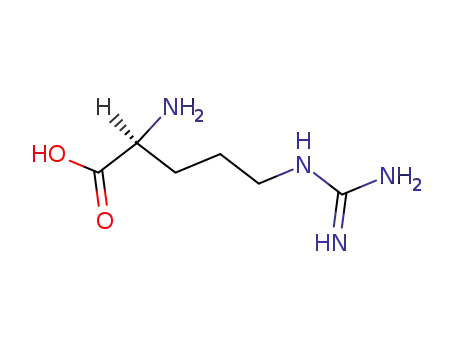
-
74-79-3,25212-18-4
L-arginine

-

-
372-75-8
Citrulline
| Conditions | Yield |
|---|---|
|
for 46 - 120h;
Product distribution / selectivity;
Enzymatic reaction;
|
91% |
|
With
dihydrogen peroxide;
K[Ru(Hedta)Cl]*2H2O;
|
71% |
|
durch Einwirkung von Faeulnisbakterien;
|
|
|
durch Einwirkung von Pseudomonas aeruginosa (Bacillus pyocyaneus);
|
|
|
durch Einwirkung von Streptococcus faecalis;
|
|
|
With
recombinant neuronal nitric oxide synthase;
Further Variations:;
Reagents;
Enzyme kinetics;
|
|
|
With
arginine deiminase; potassium 2-(N-morpholino)ethanesulfonate; magnesium chloride;
at 25 ℃;
pH=5.6;
Enzyme kinetics;
|
|
|
With
neuronal nitric oxide synthase μ; NADPH; calmodulin;
at 25 ℃;
Further Variations:;
Reaction partners;
Reagents;
pH-values;
Enzyme kinetics;
|
|
|
With
magnesium chloride;
In
various solvent(s);
at 25 ℃;
pH=5.6;
Enzyme kinetics;
|
|
|
With
glutamate dehydrogenase; 1,4-dihydronicotinamide adenine dinucleotide;
In
various solvent(s);
at 25 ℃;
pH=7.0;
Enzyme kinetics;
|
|
|
With
magnesium chloride;
In
various solvent(s);
at 25 ℃;
pH=5.6;
Enzyme kinetics;
|
|
|
With
magnesium chloride;
In
various solvent(s);
at 25 ℃;
pH=7.5;
Enzyme kinetics;
|
|
|
With
magnesium chloride;
In
various solvent(s);
at 25 ℃;
pH=6.0;
Enzyme kinetics;
|
|
|
With
full-length inducible nitric oxide synthase; (6R)-5,6,7,8-tetrahydro-L-biopterin; oxygen;
at 37 ℃;
pH=7.4;
Kinetics;
aq. buffer;
|
|
|
With
1,4-dithio-D,L-threitol; human neuronal nitric oxide synthase; NADPH; calcium chloride; hemoglobin; calmodulin; (6R)-L-erythro-5,6,7,8-tetrahydrobiopterin;
at 20 ℃;
pH=7.4;
Concentration;
Reagent/catalyst;
Kinetics;
aq. Hepes buffer;
Enzymatic reaction;
|
|
|
With
α-ketoglutaric acid; bovine liver glutamate dehydrogenase type II; recombinant Giardia lamblia arginine deiminase; water;
at 25 ℃;
pH=7.5;
pH-value;
Reagent/catalyst;
Time;
Kinetics;
aq. buffer;
|
|
|
With
arginine deiminase K5T/D44E/H404R mutant;
at 37 ℃;
Reagent/catalyst;
pH-value;
Kinetics;
aq. phosphate buffer;
|
|
|
With
1,4-dithio-D,L-threitol; neuronal NO synthase; NADPH; FAD; 4-(2-hydroxyethyl)-1-piperazinepropanesulfonic acid; (6-R,S)-5,6,7,8-tetrahydro-L-biopterin dihydrochloride; Flavin mononucleotide; superoxide dismutase; catalase; calmodulin;
at 25 ℃;
Enzymatic reaction;
|
|
|
With
hydrogenchloride; water;
at 38 ℃;
for 96h;
pH=6;
|
372-75-8 Upstream products
-
70-26-8
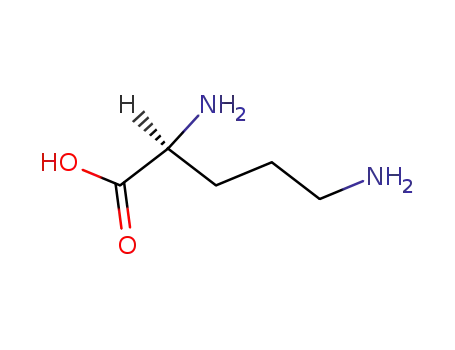
L-ornithine
-
74-79-3

L-arginine
-
1188-38-1
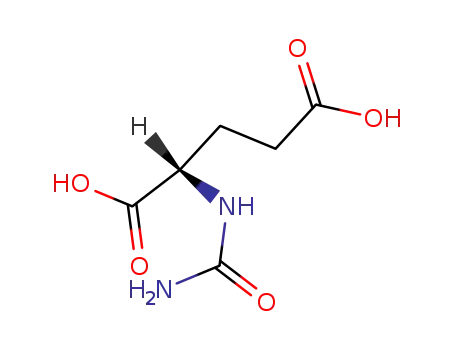
L-carglumic acid
-
1119-34-2
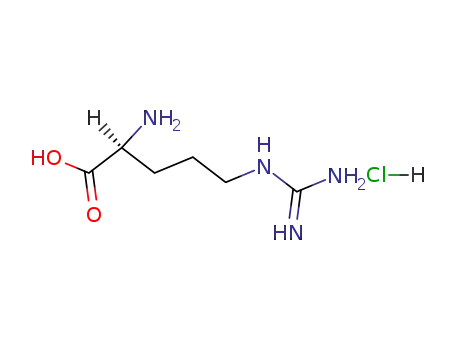
L-arginine hydrochloride
372-75-8 Downstream products
-
26117-20-4
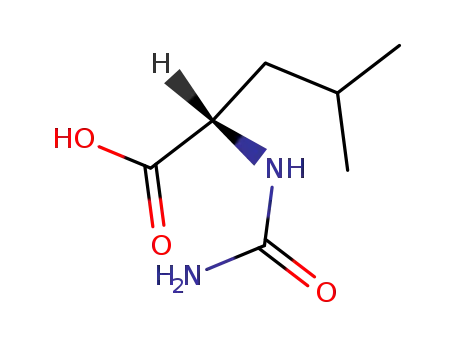
N-carbamoyl-L-leucine
-
37534-65-9
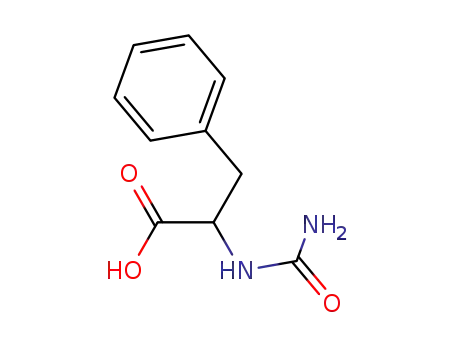
D-2-carbamoylamino-3-phenylpropionic acid
-
74-79-3

L-arginine
-
6692-89-3
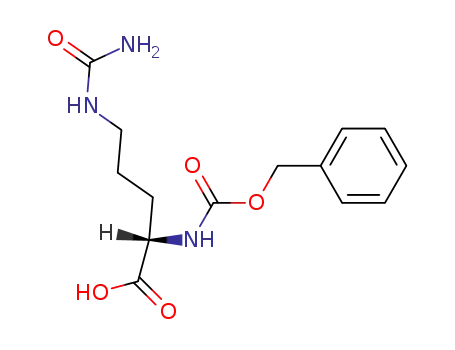
Nα-benzyloxy-carbonyl-L-citrulline
Relevant Products
-
Tesamorelin
CAS:218949-48-5
-
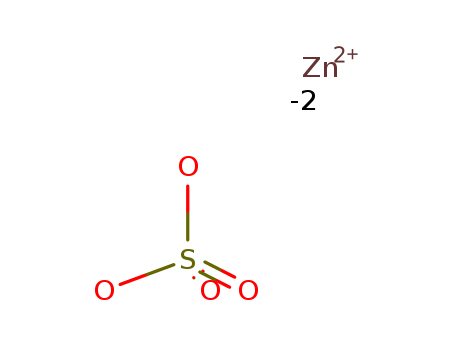
Zinc sulphate
CAS:7733-02-0
-
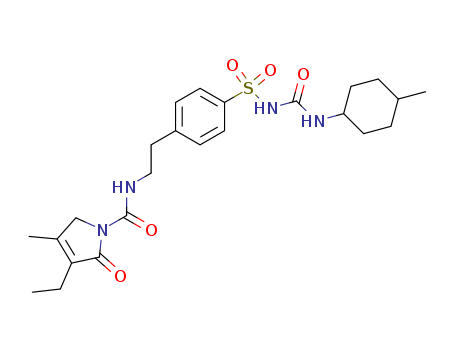
Glimepiride
CAS:93479-97-1