7235-40-7
- Product Name:beta-Carotene
- Molecular Formula:C40H56
- Purity:99%
- Molecular Weight:536.885
Product Details;
CasNo: 7235-40-7
Molecular Formula: C40H56
Appearance: red to purple power
Factory Supply Industrial Grade beta-Carotene 7235-40-7 with Best Price
- Molecular Formula:C40H56
- Molecular Weight:536.885
- Appearance/Colour:red to purple power
- Vapor Pressure:2.71E-16mmHg at 25°C
- Melting Point:176-184 °C (dec.)
- Refractive Index:1.565
- Boiling Point:654.7 °C at 760 mmHg
- Flash Point:346 °C
- PSA:0.00000
- Density:0.941 g/cm3
- LogP:12.60580
beta-Carotene(Cas 7235-40-7) Usage
|
Precursor of Vitamin A (Retinol) |
Beta-carotene is enzymatically cleaved in the intestinal mucosa to form two molecules of vitamin A (retinol), making it an important provitamin A compound. Vitamin A is essential for various bodily functions, including vision, immune system function, and skin health. |
|
Antioxidant Properties |
Beta-carotene acts as an antioxidant, helping to neutralize activated oxygen molecules that can damage cells. Its antioxidant properties contribute to protecting cells from oxidative stress and reducing the risk of chronic diseases associated with oxidative damage. |
|
Cancer Prevention |
Dietary intake of beta-carotene-containing foods has been associated with cancer prevention. Beta-carotene's antioxidant properties may help reduce the risk of certain types of cancer by neutralizing free radicals and protecting cells from damage. |
|
Food Industry Applications |
Beta-carotene is used in the food industry as a natural pigment and nutrient additive. It provides color to various food products and serves as a source of provitamin A, enhancing the nutritional value of processed foods. |
|
Nutritional Benefits |
Beta-carotene is an important nutrient for human health, contributing to overall well-being and functioning. Despite low absorption from natural sources, beta-carotene intake from foods is generally considered beneficial and safe. |
|
Synthesis and Extraction Methods |
Beta-carotene can be obtained through various methods, including extraction from natural resources (plants or algae), chemical synthesis, biosynthesis, and genetic engineering. Extraction methods include organic solvent extraction, ultrasonic wave extraction, supercritical fluid extraction (SFE), enzymatic extraction, and innovative synthesis techniques. |
|
Potential Role in Cognitive Health |
Recent research suggests a potential association between beta-carotene intake and the maintenance of cognitive function. Beta-carotene, either alone or in combination with other dietary compounds, may play a role in maintaining mental health and cognitive performance, possibly acting synergistically with other micronutrients. |
|
Definition |
ChEBI: A cyclic carotene obtained by dimerisation of all-trans-retinol. A strongly-coloured red-orange pigment abundant in plants and fruit and the most active and important provitamin A carotenoid. |
|
Manufacturing Process |
3.6 g (0.023 mol) of 3,8-dimethyl-3,5,7-decatrien-1,9-diyne were dissolved in 50 ml of absolute ether, and to the solution was added 0.05 mol of ethereal phenyl-lithium solution. The mixture was refluxed for 30 minutes. Then a solution of 11 g (0.05 mol) of 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2-methyl- 2-buten-1-al in 100 ml of ether was added dropwise, and the reaction mixture was boiled for 2 hours. The reaction mixture was then hydrolyzed with aqueous ammonium acetate solution, and the ethereal layer was separated, dried and concentrated. The residue, i.e., 1,18-di(2,6,6-trimethyl-1- cyclohexen-1-yl)-3,7,12,16-tetramethyl-4,15-dihydroxy-2,7,9,11,16- octadecapentaen-5,13-diyne, was a resinous product (having 1.9 active hydrogen atoms and absorption maxima in the ultraviolet spectrum at 326 and 341 nm) which was used for the next step without any further purification. The resin was dissolved in 200 ml of methylene chloride, 10 ml of glacial acetic acid were added to the solution, and the mixture was cooled to - 40°C in a carbon dioxide atmosphere, while stirring. Then, 9 ml of aqueous hydrobromic acid (60%) were added in one portion, the mixture was stirred at -35°C for 1.5 minutes, and subsequently 200 ml of ice water were run into the mixture. After further stirring the mixture for 2 hours at 0°C, the methylene chloride layer was separated, washed with water and sodium bicarbonate solution, dried with Na2SO4 andconcentrated in vacuo. The residue, i.e., 11,12-11',12'-bisdehydro-betta-carotene, was a tough resin or a foamy solid (having no active hydrogen atoms and possessing absorption maxima in the ultraviolet spectrum at 334 and 408 nm). This product can be purified by chromatography. The crude product can also be used for the next step without any preliminary purification.11.4 g of 11,12-11',12'-bisdehydro-β-carotene were dissolved in 100 ml of petroleum ether (boiling range 80° to100°C), and the solution was hydrogenated under normal conditions after the addition of 0.5 ml of quinoline and 5 g of a lead-poisoned palladium catalyst. After the calculated amount of hydrogen had been absorbed, the catalyst was removed by filtration and the filtrate was extracted with dilute sulfuric acid to remove the quinoline. By concentrating the solution in the usual manner there was obtained 11,12- 11',12'-di-cis-carotene. The product was purified by recrystallization from benzene-alcohol. The purified product melts at 154°C; absorption maxima in the ultraviolet spectrum at 276, 334, 338, 401 and 405 nm. The isomerization was effected by heating the product for 10 hours at 90 to 100°C in high-boiling petroleum ether in a carbon dioxide atmosphere. The resulting and carotene melted at 180°C; ultraviolet absorption maxima at 452 and 480 nm.Preparation of the intermediates for the above chemical synthesis are also described in US. Patent 2,917,539. The other patents cited below describe a fermentation route. US Patent 2,848,508 describes preparation from carrots. |
|
Brand name |
BetaVit (BASF); Lucaratin (BASF); Solatene (Hoffmann-LaRoche). |
|
Therapeutic Function |
Vitamin A precursor, Sunscreen agent |
|
General Description |
beta-Carotene is an antioxidant and is one of the most important carotenoids and a source of vitamin A. It is abundantly present in fruits and vegetables which is also used as a food supplement and a colorant. |
|
Biochem/physiol Actions |
The most important of the provitamins A, β-carotene can be classified as an antioxidant due to its inhibition of radical initiated peroxidation in vitro. However, in vivo it appears to act either as an antioxidant or a prooxidant depending on cellular environment. It reduces the incidence of many cancers, but enhances lung cancer incidence in smokers. |
|
Safety Profile |
When heated to decomposition it emits acrid smoke and irritating fumes. |
|
Purification Methods |
It forms purple prisms when crystallised from *C6H6/MeOH and red rhombs from pet ether. Its solubility in hexane is 0.1% at 0o. It is oxygen sensitive and should be stored under N2 at -20o in the dark. It gives a deep blue colour with λmax at 590nm when mixed with SbCl3 in CHCl3. UV: (*C6H6) 429infl, max at 454 and 484nm. The principal peak at 454nm has 1cm 1% 2000. [Synthesis: Surmatis & Ofner J Org Chem 26 1171 1961; Milas et al. J Am Chem Soc 72 4844 1950.] β-Carotene is also purified by column chromatography (Al2O3 activity I-II). It is dissolved in pet ether/*C6H6 (10:1), applied to the column and eluted with pet ether/EtOH; the desired fraction is evaporated and the residue is recrystallised from *C6H6/MeOH (violet-red plates). [UV: Inhoffen et al. Justus Liebigs Ann Chem 570 54, 68 1950; Review: Fleming Selected Organic Synthesis (J Wiley, Lond) pp. 70-74 1973.] Alternatively it can be purified by chromatography on a magnesia column, thin layer of Kieselguhr or magnesia. Crystallise it from CS2/MeOH, Et2O/pet ether, acetone/pet ether or toluene/MeOH. Store it in the dark, under an inert atmosphere, at -20o. Recrystallise it also from 1:1 EtOH/CHCl3. [Bobrowski & Das J Phys Chem 89 5079 1985, Johnston & Scaiano J Am Chem Soc 1 0 8 2349 1986, Strain J Biol Chem 105 523 1934, Meth Biochem Anal 4 1 1957, Beilstein 5 II 638, 5 III 2453, 5 IV 2617.] |
InChI:InChI=1/C40H56/c1-31(19-13-21-33(3)25-27-37-35(5)23-15-29-39(37,7)8)17-11-12-18-32(2)20-14-22-34(4)26-28-38-36(6)24-16-30-40(38,9)10/h11-14,17-22,25-28H,15-16,23-24,29-30H2,1-10H3/b12-11+,19-13+,20-14+,27-25+,28-26u,31-17-,32-18+,33-21-,34-22+
7235-40-7 Relevant articles
Baicalin in radical scavenging and its synergistic effect with β-carotene in antilipoxidation
Liang,Rui-Min,Li-Min,Xi-Cheng,Zhang, Jian-Ping,Skibsted, Leif H.
, p. 7118 - 7124 (2009)
The lipophilic flavonoid glycoside baica...
Preparation method of β - carotene with high total trans content
-
Paragraph 0063; 0065; 0085-0086, (2021/09/01)
The invention provides a preparation met...
Method for preparing beta-carotene
-
Paragraph 0053-0056, (2021/02/04)
The invention relates to a compound prep...
Bidirectional Hiyama–Denmark Cross-Coupling Reactions of Bissilyldeca-1,3,5,7,9-pentaenes for the Synthesis of Symmetrical and Non-Symmetrical Carotenoids
Rivas, Aurea,Pérez-Revenga, Víctor,Alvarez, Rosana,de Lera, Angel R.
, p. 14399 - 14407 (2019/11/03)
The construction of the carotenoid skele...
7235-40-7 Process route
-
marigold oleoresin
-
marigold oleoresin

-
cis-luteins
-
cis-luteins

-
-
7235-40-7
beta-carotene

-

-
144-68-3,3309-19-1,24502-92-9,29472-68-2,31272-50-1,31272-53-4,57820-80-1,60046-54-0,60497-64-5,60497-65-6,72002-36-9,72580-10-0,100347-92-0,100347-97-5,136656-95-6,136733-98-7,136733-99-8,136734-01-5,136734-02-6
zeaxanthin

-

-
127-40-2
lutein

-
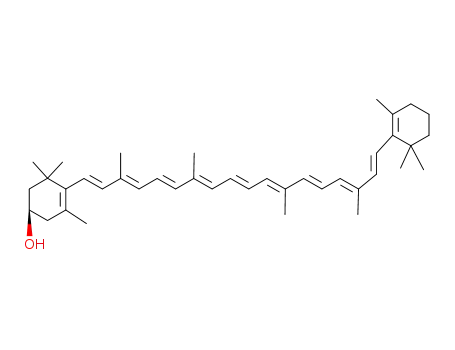
-
472-70-8
(3R)-cryptoxanthin
| Conditions | Yield |
|---|---|
|
With
potassium hydroxide; water;
In
ethanol;
at 73 - 75 ℃;
for 0.5h;
Product distribution / selectivity;
|
-
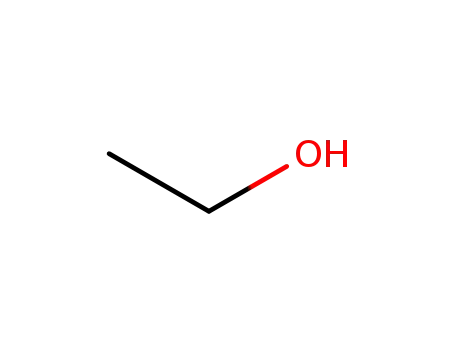
-
64-17-5
ethanol

-
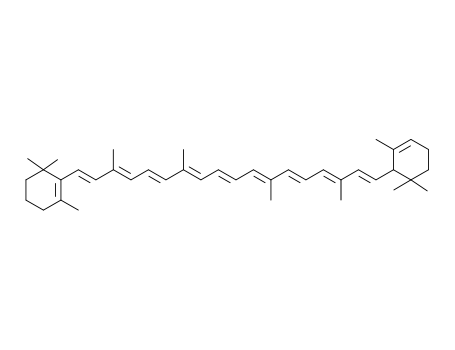
-
7488-99-5
alpha-carotene

-
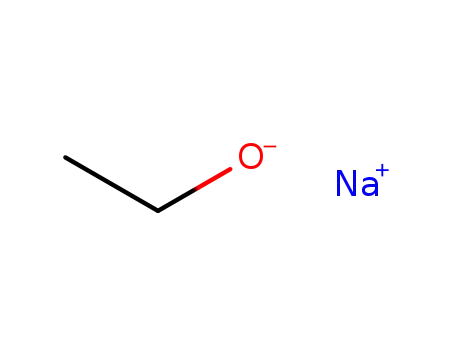
-
141-52-6
sodium ethanolate

-
-
71-43-2,26181-88-4,54682-86-9,13967-78-7,174973-66-1
benzene

-

-
7235-40-7
beta-carotene
| Conditions | Yield |
|---|---|
|
at 100 - 110 ℃;
(+)-all-trans-α-carotene;
|
7235-40-7 Upstream products
-
110-54-3
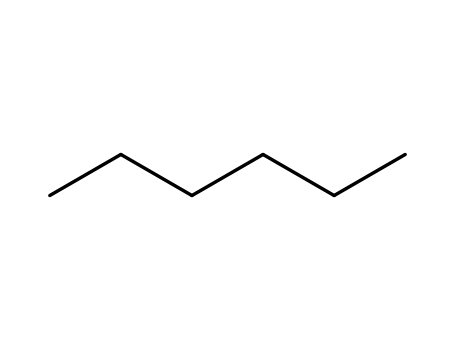
hexane
-
19361-58-1
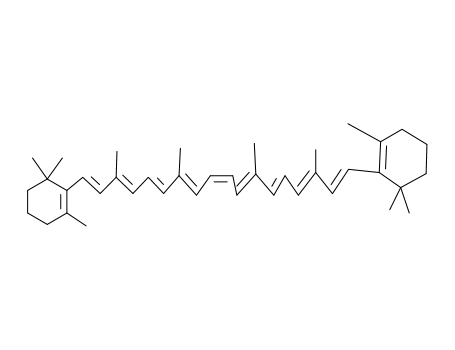
15-cis-β-carotene
-
141-52-6

sodium ethanolate
-
13312-52-2
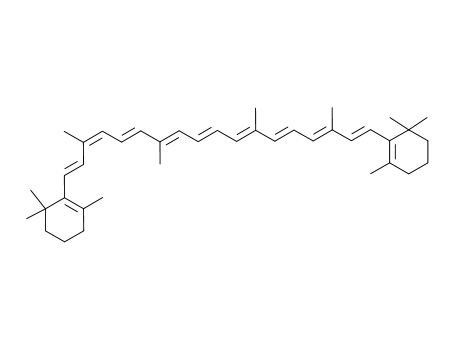
9-cis-β-carotene
7235-40-7 Downstream products
-
514-78-3
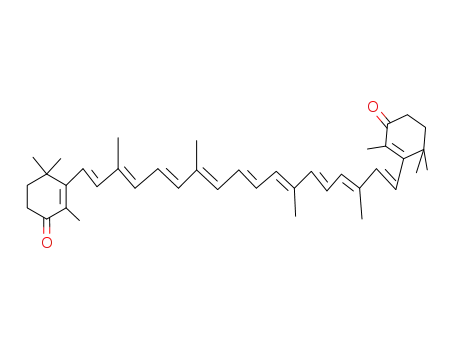
canthaxanthin
-
432-68-8

echinenone
-
1923-89-3
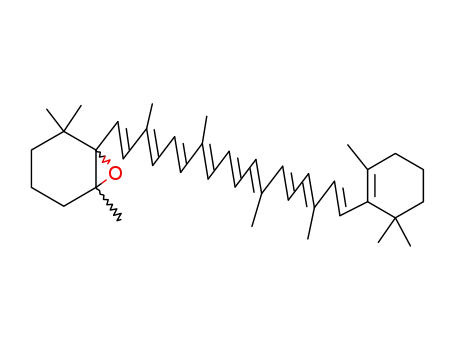
5,6-epoxy-5,6-dihydro-β,β-carotene
-
116-31-4
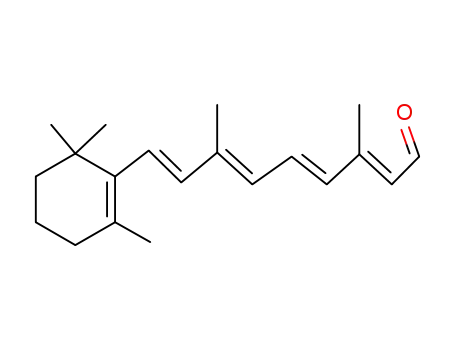
all-trans-Retinal
Relevant Products
-
Tesamorelin
CAS:218949-48-5
-
5-Fluorouracil
CAS:51-21-8
-
Matrine
CAS:519-02-8