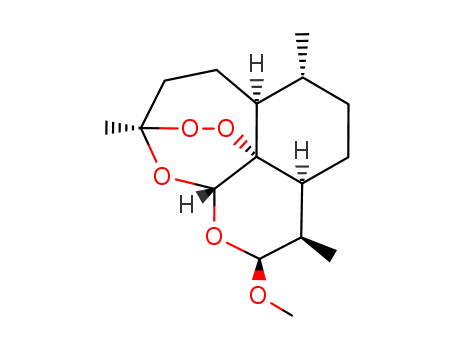
71963-77-4
- Product Name:Artemether
- Molecular Formula:C16H26O5
- Purity:99%
- Molecular Weight:298.379
Product Details;
CasNo: 71963-77-4
Molecular Formula: C16H26O5
Quality Factory Hot Selling Artemether 71963-77-4 with Fast Shipping
- Molecular Formula:C16H26O5
- Molecular Weight:298.379
- Melting Point:86-89oC
- Refractive Index:1.518
- Boiling Point:357.5 °C at 760 mmHg
- Flash Point:140.5 °C
- PSA:46.15000
- Density:1.18 g/cm3
- LogP:2.84080
Artemether(Cas 71963-77-4) Usage
|
Definition |
ChEBI: An artemisinin derivative that is artemisinin in which the lactone has been converted to the corresponding lactol methyl ether. It is used in combination with lumefantrine as an antimalarial for the treatment of multi-drug resistant strains of falcip rum malaria. |
|
General Description |
Artemisinin (ART) is a natural compound present in Artemisia annua, a traditional Chinese plant. |
|
Biochem/physiol Actions |
Artemether is a methyl ether derivative of artemisinin. It is used against multi-drug resistant strains of the malaria parasite, Plasmodium falciparum, and shows potential in treatment of schistosomiasis. |
|
Safety Profile |
Poison by intramuscular route.Experimental reproductive effects. When heated todecomposition it emits acrid smoke and irritating fumes. |
InChI:InChI=1/C16H26O5/c1-9-5-6-12-10(2)13(17-4)18-14-16(12)11(9)7-8-15(3,19-14)20-21-16/h9-14H,5-8H2,1-4H3/t9-,10-,11+,12+,13+,14-,15-,16?/m1/s1
71963-77-4 Relevant articles
One-pot green synthesis of β-artemether/arteether
Kumar, Atul,Bishnoi, Ajay Kumar
, p. 31973 - 31976 (2014)
An efficient one pot green synthesis of ...
Preparation method of beta-artemether
-
Paragraph 0046-0072, (2021/02/13)
The invention belongs to the field of or...
A process for preparing β - pedic ether process
-
Paragraph 0023-0024; 0025-0026; 0027-0028, (2018/04/21)
The invention discloses a technology for...
Process for preparation of β - pedic methyl ether
-
Paragraph 0041-0044; 0067; 0068, (2017/08/25)
The invention relates to a preparation m...
Method for producing artesunate
-
Paragraph 0012-0036, (2017/06/06)
The invention relates to a method for pr...
71963-77-4 Process route
-
-
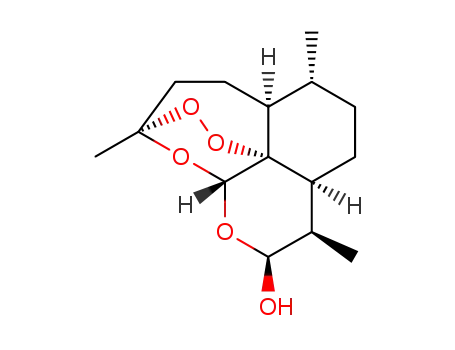
71939-50-9
dihydroartemisinin

-
-
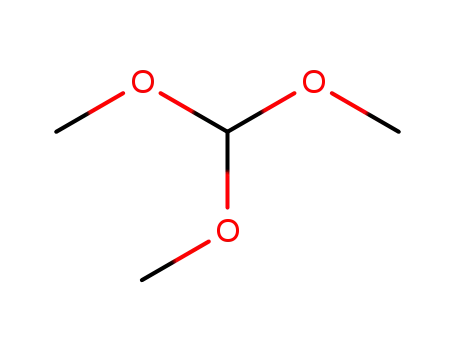
149-73-5
trimethyl orthoformate

-
-
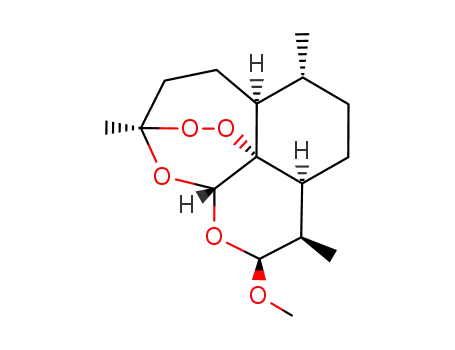
71963-77-4
artemether
| Conditions | Yield |
|---|---|
|
With
methanesulfonic acid;
In
ethanol;
at 5 - 20 ℃;
for 3h;
pH=5;
Solvent;
pH-value;
Temperature;
Reagent/catalyst;
|
96.6% |
|
toluene-4-sulfonic acid;
In
methanol;
at 30 ℃;
for 0.5h;
|
-
-
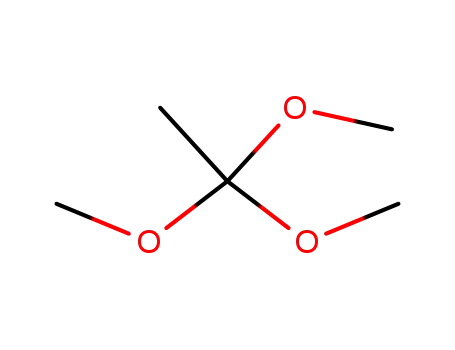
1445-45-0
Trimethyl orthoacetate

-

-
71939-50-9
dihydroartemisinin

-

-
71963-77-4
artemether
| Conditions | Yield |
|---|---|
|
With
perchloric acid;
In
ethanol;
at 0 ℃;
for 8h;
pH=2;
Reagent/catalyst;
pH-value;
Temperature;
|
97.6% |
71963-77-4 Upstream products
-
53562-86-0
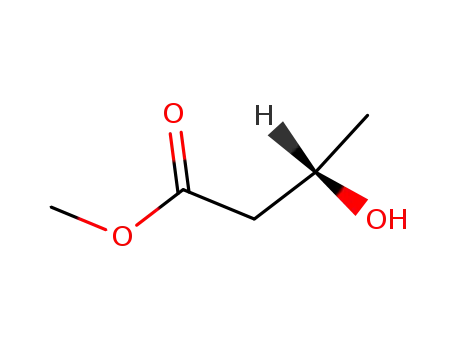
(S)-3-hydroxybutyric acid methyl ester
-
71939-50-9
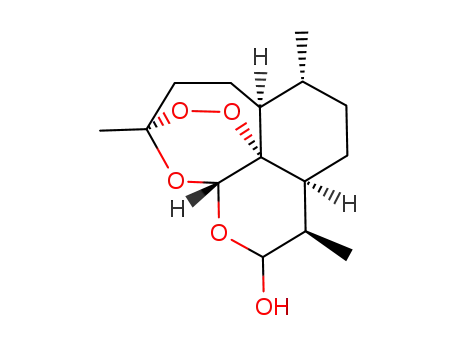
dihydroartemisinin
-
3976-69-0
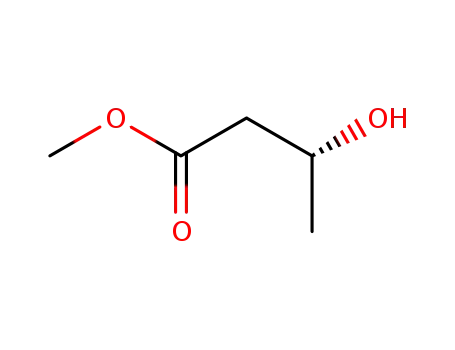
Methyl (R)-3-hydroxybutyrate
-
67-56-1
methanol
71963-77-4 Downstream products
-
181528-64-3
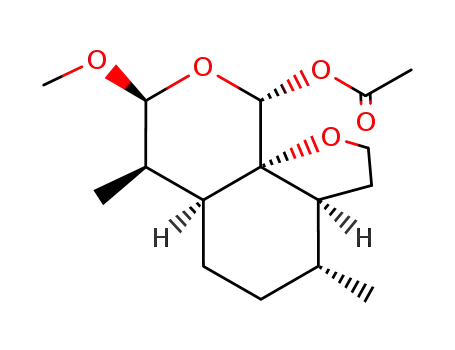
9α-acetoxy-10β-methoxyartemethin-I
-
174097-70-2
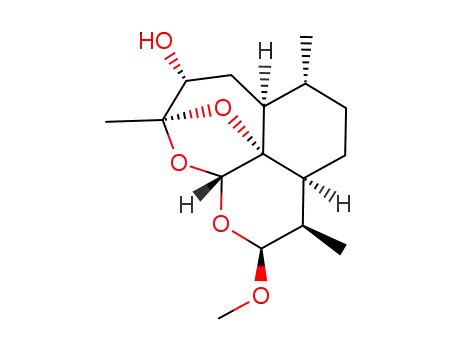
3α-hydroxy-12β-methoxyartemethin-III
-
1356857-18-5
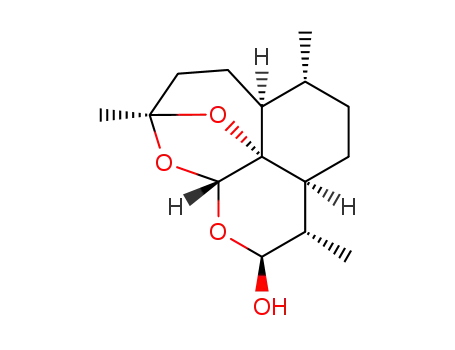
β-2-deoxy-9-epidihydroartemisinin
-
107466-88-6
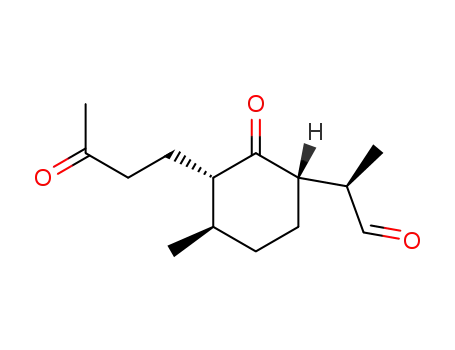
(2S, 3R, 6S)-2-(3-oxobutyl)-3-methyl-6-<(R)2-propanal>-cyclohexanone
Relevant Products
-
Tesamorelin
CAS:218949-48-5
-
Propylparaben
CAS:94-13-3
-
Sermorelin Acetate
CAS:86168-78-7