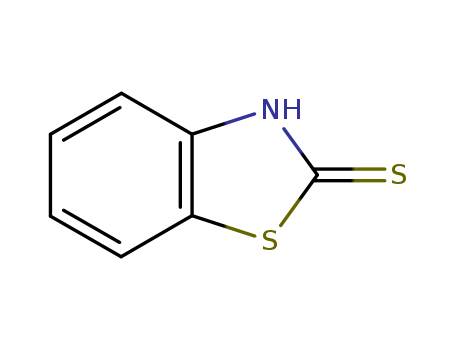
149-30-4
- Product Name:2-Mercaptobenzothiazole
- Molecular Formula:C7H5NS2
- Purity:99%
- Molecular Weight:167.255
Product Details;
CasNo: 149-30-4
Molecular Formula: C7H5NS2
Appearance: beige or light yellow powder with a faint odour
Reputable factory supply 2-Mercaptobenzothiazole 149-30-4 in stock with high standard
- Molecular Formula:C7H5NS2
- Molecular Weight:167.255
- Appearance/Colour:beige or light yellow powder with a faint odour
- Vapor Pressure:0.000844mmHg at 25°C
- Melting Point:177-181 °C(lit.)
- Refractive Index:1.783
- Boiling Point:305 °C at 760 mmHg
- PKA:9.80±0.20(Predicted)
- Flash Point:138.3 °C
- PSA:79.93000
- Density:1.46 g/cm3
- LogP:2.58500
2-Mercaptobenzothiazole(Cas 149-30-4) Usage
|
Chemical Description |
2-mercaptobenzothiazole was used in the preparation of unprotected (aminoacyl)amino nucleosides, oxalic acid was added to the solution to give the nucleosides as a white powder, and silica gel was used in the purification of the residue. |
|
Preparation |
2-Mercaptobenzothiazole is produced by reacting aniline, carbon disulfide, and sulfur at high temperature and pressure; the product is then purified by dissolution in a base to remove the dissolved organics. Re-precipitation is achieved by the addition of acid (Kirk-Othmer, 1982; NTP, 1988).Refined 2-mercaptobenzothiazole was produced by recrystallization from 2-mercaptobenzothiazole with industrial grade and oxidized to 2,2'-dithiobis(benzothiazole), using oxygen as an oxidant, nitric oxide as a oxygen carrier and alcohols as solvents, in a circulating fluidized reactor under one-step oxidation. 2,2'-Dithiobis(benzothiazole) was thus obtained with high purity up to 99 %, melting point at 183 oC, high yield over 98 %, through the optimization of reaction parameters as reaction time, temperature, reactants ratio, with less waste generation and emission during the production process. Alcohol solvents can be reused after purification.http://dx.doi.org/10.14233/ajchem.2013.14030 |
|
Synthesis Reference(s) |
The Journal of Organic Chemistry, 26, p. 3436, 1961 DOI: 10.1021/jo01067a101 |
|
Air & Water Reactions |
Insoluble in water. |
|
Reactivity Profile |
2-Mercaptobenzothiazole is incompatible with strong oxidizing agents. Also incompatible with acids and acid fumes. |
|
Health Hazard |
Thiazoles cause allergic skin reactions of type IV [delayed-type hypersensitivity (DTH)]. 2-mercaptobenzothiazole is a Standardized Chemical Allergen. The physiologic effect of 2-mercaptobenzothiazole is by means of Increased Histamine Release, and Cell-mediated Immunity. |
|
Fire Hazard |
2-Mercaptobenzothiazole is combustible. |
|
Safety Profile |
Suspected carcinogen withexperimental carcinogenic and tumorigenic data. Poisonby ingestion and intraperitoneal routes. Experimentalteratogenic and reproductive effects. Mutation datareported. Incompatible with oxidizers. When heated todecomposition |
|
Carcinogenicity |
MBT was not mutagenic in Ames bacterial assays, but it induced chromosomal damage in mammalian cells in culture. Reproductive effects were not observed in two-generation studies of rats treated with up to 15,000 ppm MBT in the diet. |
|
Purification Methods |
Crystallise it repeatedly from 95% EtOH, or purify it by incomplete precipitation by dilute H2SO4 from a basic solution, followed by several crystallisations from acetone/H2O or *benzene. It complexes with Ag, Au, Bi, Cd, Hg, Ir, Pt, and Tl. [Beilstein 27 II 233, 27 III/IV 2709.] |
|
General Description |
2-Mercaptobenzothiazole (MBT) is a versatile chemical compound primarily used as a vulcanization accelerator in rubber production. It also serves as a key intermediate in the synthesis of biologically active derivatives, such as amides, anilides, hydrazones, and pyridine derivatives, which have shown potential anticonvulsant and monoamine oxidase inhibitory properties. Its reactivity with various reagents enables the formation of structurally diverse compounds, making it valuable in pharmaceutical and industrial applications. |
|
Application |
2-mercaptobenzothiazole is an accelerator, retarder, and peptizer for natural and other rubber products, but is also used as a corrosion inhibitor in soluble cutting oils and antifreeze mixtures; in greases, adhesives, photographic-film emulsions; detergents; veterinary products, such as tick and flea powders and sprays.It is added to polyether polymers as a stabilizer to resist damage by air and ozone, and is a component approved in the USA in some skin medications for dogs (HSDB, 2015).2-Mercaptobenzothiazole is also used as an intermediate in the production of pesticides such as 2-(thiocyanomethylthio)benzothiazole (Azam & Suresh, 2012), and sodium and zinc salts of 2-mercaptobenzothiazole are approved for use as pesticides by the EPA (1994). |
|
Definition |
ChEBI: 2-Mercaptobenzothiazole is a 1,3-Benzothiazole substituted at the 2-position with a sulfanyl group. It is used as a vulcanisation accelerator in the crosslinking of rubber. |
InChI:InChI=1/C7H5NS2/c9-7-8-5-3-1-2-4-6(5)10-7/h1-4H,(H,8,9)
149-30-4 Relevant articles
Design, synthesis and biological evaluation of bivalent benzoxazolone and benzothiazolone ligands as potential anti-inflammatory/analgesic agents
Abdelazeem, Ahmed H.,Khan, Shabana I.,White, Stephen W.,Sufka, Kenneth J.,McCurdy, Christopher R.
, p. 3248 - 3259 (2015)
Abstract Benzoxazolone and benzothiazolo...
-
Watt
, p. 436,437, 439 (1939)
-
-
Dunbrook,Zimmermann
, p. 2734 (1934)
-
A convenient metal-free method for the synthesis of benzothiazolethiones from o-haloanilines and carbon disulfide
Wang, Fei,Xi, Chanjuan,Zhao, Peng
, p. 1477 - 1480 (2012)
A convenient method has been developed f...
9-Fluorenylmethyl (Fm) Disulfides: Biomimetic Precursors for Persulfides
Park, Chung-Min,Johnson, Brett A.,Duan, Jicheng,Park, Jeong-Jin,Day, Jacob J.,Gang, David,Qian, Wei-Jun,Xian, Ming
, p. 904 - 907 (2016)
The development of a functional disulfid...
A Highly Efficient Synthesis of 2-Benzimidazolthiones and Their Congeners under Mild Conditions
Li, Wei-wei,Zheng, Hui
, p. 175 - 181 (2019)
-
PdCl2/DMSO-Catalyzed Thiol-Disulfide Exchange: Synthesis of Unsymmetrical Disulfide
Guo, Jimin,Zha, Jianjian,Zhang, Tao,Ding, Chang-Hua,Tan, Qitao,Xu, Bin
supporting information, p. 3167 - 3172 (2021/05/05)
Unsymmetrical disulfides have been effec...
Purification of high-purity 2-mercaptobenzothiazole by two-steps
Zhao, Zengbing,Chen, Bo,Cheng, Lanxing,Zhao, Yili,Chai, Yongli,Yang, Shucheng
, p. 851 - 859 (2021/05/19)
High-purity 2-mercaptobenzothiazole (2-M...
Benzo[d]thiazole-2-thiol bearing 2-oxo-2-substituted-phenylethan-1-yl as potent selectivelasBquorum sensing inhibitors of Gram-negative bacteria
Quoc, Thang Nguyen,Thanh, Tung Truong,Xuan, Huy Luong
, p. 28797 - 28808 (2021/09/22)
Quorum sensing is a well-known term for ...
A new strategy for the synthesis of 2-mercaptobenzazole derivatives by green chemistry metrics
Vessally, Esmail,Monfared, Aazam,Eskandari, Zahra,Abdoli, Morteza,Hosseinian, Akram
supporting information, p. 1 - 5 (2020/08/25)
A green and efficient method has been de...
149-30-4 Process route
-
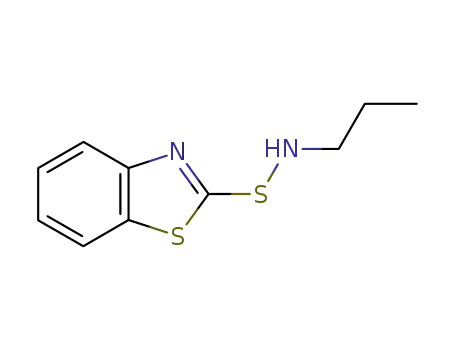
-
66552-53-2
N-propyl-2-benzothiazolesulfenamide

-
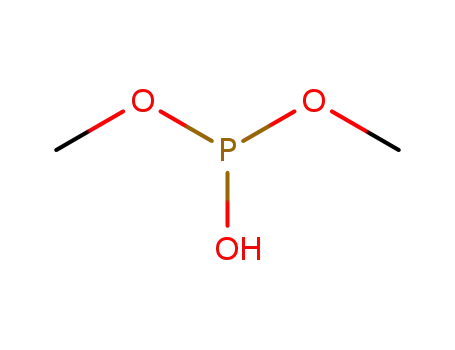
-
96-36-6,868-85-9
methyl phosphite

-
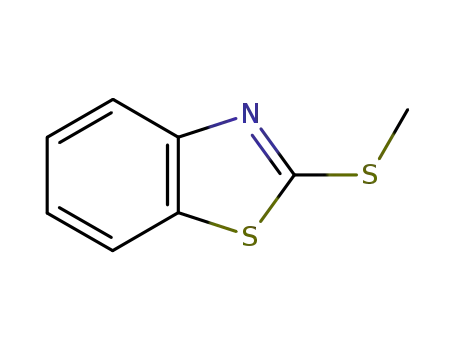
-
615-22-5
2-methylmercaptobenzothiazole

-
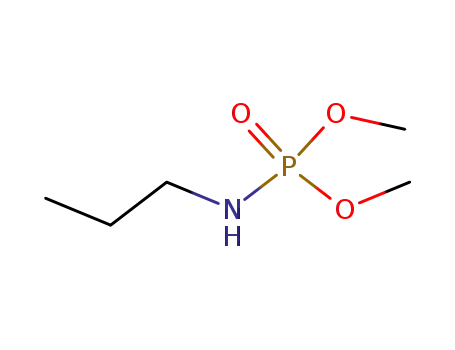
-
20465-00-3
N-Propyl-O,O-dimethyl-phosphorsaeureamid

-
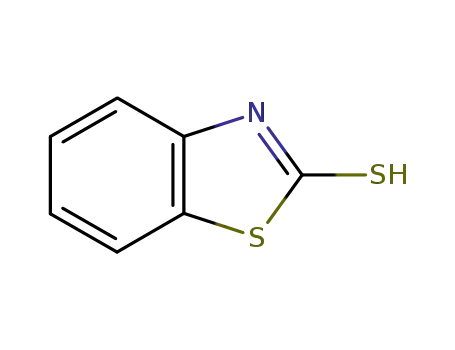
-
149-30-4,118090-09-8
2-Mercaptobenzothiazole
| Conditions | Yield |
|---|---|
|
|
80% 5% 95% |
|
|
80% 90% 5% |
-

-
96-36-6,868-85-9
methyl phosphite

-
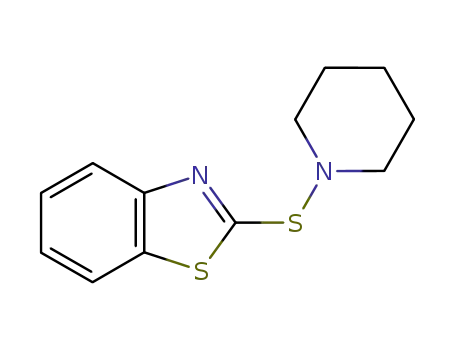
-
26773-65-9
N-piperidin-2-benzothiazole sulfenamide

-

-
615-22-5
2-methylmercaptobenzothiazole

-
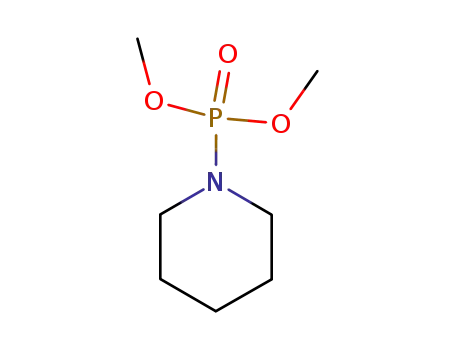
-
597-24-0
Dimethyl piperididophosphate

-

-
149-30-4,118090-09-8
2-Mercaptobenzothiazole
| Conditions | Yield |
|---|---|
|
|
93% 93% 7% |
149-30-4 Upstream products
-
1155-00-6
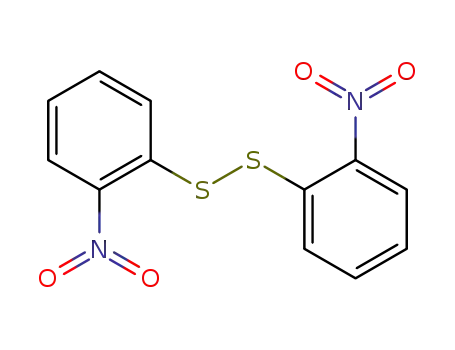
bis(2-nitrophenyl)disulfide
-
95-16-9
1,3-Benzothiazole
-
109-57-9
Allylthiourea
-
615-20-3
2-chlorobenzo[d][1,3]thiazole
149-30-4 Downstream products
-
26773-65-9

N-piperidin-2-benzothiazole sulfenamide
-
102-77-2

4-(benzothiazole-2-sulfenyl)-morpholine
-
93963-17-8
1,4-bis-(benzothiazole-2-sulfenyl)-piperazine
-
102-78-3

2-(2,6-dimethyl-morpholin-4-ylsulfanyl)-benzothiazole
Relevant Products
-
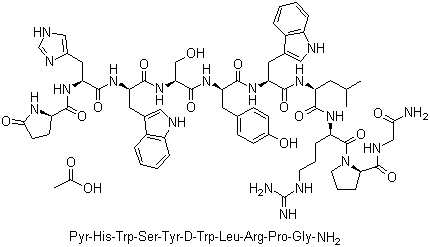
Tesamorelin
CAS:218949-48-5
-
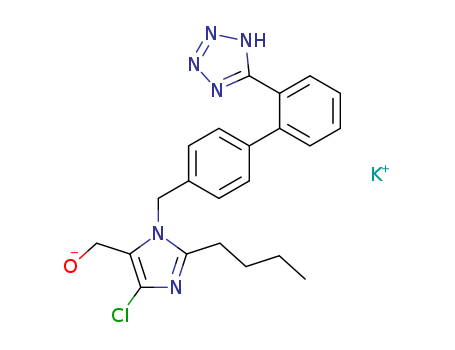
Losartan potassium
CAS:124750-99-8
-
Copper
CAS:7440-50-8