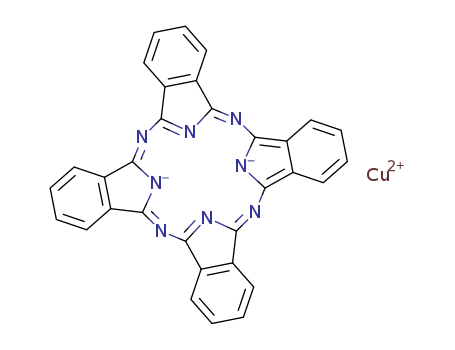
147-14-8
- Product Name:(29H,31H-phthalocyaninato(2-)-N29,N30,N31,N32)copper
- Molecular Formula:C32H16CuN8
- Purity:99%
- Molecular Weight:576.079
Product Details;
CasNo: 147-14-8
Molecular Formula: C32H16CuN8
Appearance: dark blue fine crystalline powder
Cosmetics Grade (29H,31H-phthalocyaninato(2-)-N29,N30,N31,N32)copper 147-14-8 For Sale with Good Price
- Molecular Formula:C32H16CuN8
- Molecular Weight:576.079
- Appearance/Colour:dark blue fine crystalline powder
- Melting Point:600 °C (dec.)
- PSA:84.02000
- Density:1.62[at 20℃]
- LogP:1.46190
(29H,31H-phthalocyaninato(2-)-N29,N30,N31,N32)copper(Cas 147-14-8) Usage
|
Application |
Copper(II) phthalocyanine, known as CuPc, has been used as an electron donor with fullerene-C60 or phenyl-C61-butyric acid methyl ester (PCBM) in vacuum-deposited organic photovoltaics (OPV). Power conversion efficiency of about 1% has been achieved [2] and improved efficiency of 4% with pentacene-doped CuPc layer.CuPc has also been used as a hole-injection material for light-emitting diodes. It has been reported that a thin CuPc layer may effectively enhance the hole injection from the anode to the emissive-polymer layer, resulting in a dramatic decrease of operating voltage of the device. Device stability was achieved by depositing a copper phthalocyanine CuPc hole-injection layer HIL on the ITO anode. The improved stability could be contributed to the good match of its highest-occupied molecular orbital (HOMO) level to the work function of ITO, and the improved wetting property of organic materials on ITO. Moreover, CuPc has very weak absorption of light, with wavelengths from 400 to 500 nm, making it suitable for use in blue and green OLEDs.Effective electron-blocking was also observed for inorganic–organic hybrid perovskite solar cells when CuPc-doped spiro-OMeTAD was used as the hole-transporting layer. |
|
General Description |
Copper(II) phthalocynaine (CuPc) is a metal phthalocyanine dye that acts as a p-type semiconductor. It has a charge mobility of 10-4 cm2/Vs. It forms a chemically stable thin film that exhibits photoconductivity and catalytic activity. |
|
Flammability and Explosibility |
Notclassified |
|
Purification Methods |
Precipitate it twice from conc H2SO4 by slow dilution with water. It has also been purified by two or three sublimations at 580o in an argon flow at 300-400Pa. [Beilstein 26 III/IV 4256.] |
|
Properties and Applications |
TEST ITEMS SPECIFICATION APPEARANCE BLUE POWDER SHADE REDDISH HEAT RESISTANCE 300 °C min LIGHT FASTNESS 7-8 ACID RESISTANCE 5 ALKALI RESISTANCE 5 FASTNESS TO BLEEDING 5 OIL ABSORPTION 40-45% SPECIFIC SURFACE 29 m 2 /g DENSITY 1.60 g/cm 3 RESIDUE ON 80 MESH 5.0% max WATER SOLUBLE 1.0% max VOLATITE 105 °C 1.0% max TINTING STRENGTH 100-105 % |
|
TEST ITEMS |
SPECIFICATION |
|
APPEARANCE |
BLUE POWDER |
|
SHADE |
REDDISH |
|
HEAT RESISTANCE |
300 °C min |
|
LIGHT FASTNESS |
7-8 |
|
ACID RESISTANCE |
5 |
|
ALKALI RESISTANCE |
5 |
|
FASTNESS TO BLEEDING |
5 |
|
OIL ABSORPTION |
40-45% |
|
SPECIFIC SURFACE |
29 m 2 /g |
|
DENSITY |
1.60 g/cm 3 |
|
RESIDUE ON 80 MESH |
5.0% max |
|
WATER SOLUBLE |
1.0% max |
|
VOLATITE 105 °C |
1.0% max |
|
TINTING STRENGTH |
100-105 % |
InChI:InChI=1/C32H16N8.Cu/c1-2-10-18-17(9-1)25-33-26(18)38-28-21-13-5-6-14-22(21)30(35-28)40-32-24-16-8-7-15-23(24)31(36-32)39-29-20-12-4-3-11-19(20)27(34-29)37-25;/h1-16H;/q-2;
147-14-8 Relevant articles
Facile one-pot preparation of thermally and photochemically convertible soluble precursors of copper phthalocyanine and naphthalocyanine
Kikukawa, Yuu,Fukuda, Takamitsu,Fuyuhiro, Akira,Ishikawa, Naoto,Kobayashi, Nagao
, p. 8518 - 8520 (2011)
Soluble copper phthalocyanine (CuPc) and...
Effect of chain length on thermal conversion of alkoxy-substituted copper phthalocyanine precursors
Fukuda, Takamitsu,Kikukawa, Yuu,Tsuruya, Ryota,Fuyuhiro, Akira,Ishikawa, Naoto,Kobayashi, Nagao
, p. 11832 - 11837 (2011)
A series of dialkoxy-substituted copper ...
(Phthalocyaninato)copper(II) complexes fused with different numbers of 15-crown-5 moieties - Synthesis, spectroscopy, supramolecular structures, and the effects of substituent number and molecular symmetry
Sheng, Ning,Zhang, Yuexing,Xu, Hui,Bao, Meng,Sun, Xuan,Jiang, Jianzhuang
, p. 3268 - 3275 (2007)
Symmetrical (phthalocyaninato)copper(II)...
A Bipolar and Self-Polymerized Phthalocyanine Complex for Fast and Tunable Energy Storage in Dual-Ion Batteries
Wang, Heng-guo,Wang, Haidong,Si, Zhenjun,Li, Qiang,Wu, Qiong,Shao, Qi,Wu, Lanlan,Liu, Yu,Wang, Yinghui,Song, Shuyan,Zhang, Hongjie
, p. 10204 - 10208 (2019)
Bipolar redox organics have attracted in...
Magneto-modified catalyst on the base of nanocrystalline CuO
Yermakov,Feduschak,Sedoi,Uimin,Mysik
, p. 2445 - 2447 (2004)
The weakly oxidized nanocrystalline copp...
X-ray analysis of phthalocyanines formed in the reaction of Au-Cu and Au-Sn alloys with 1,2-dicyanobenzene
Kubiak, Ryszard,Janczak, Jan
, p. 107 - 112 (1992)
X-ray investigations of the reactions of...
Synthesis and characterization of copper phthalocyanine and tetracarboxamide copper phthalocyanine deposited mica-titania pigments
Topuz, Berna Burcu,Guenduez, Guengoer,Mavis, Bora,Colak, Uener
, p. 31 - 37,7 (2013)
Combination pigments were synthesized by...
Singly and Doubly Oxidized Phthalocyanine (pc) Rings: [Cu(pc)(ReO4)] and [Cu(pc)(ReO4)2]
Gardberg, Anna S.,Doan, Peter E.,Hoffman, Brian M.,Ibers, James A.
, (2001)
-
Facile, liquid phase preparation of copper phthalocyanine microcrystals by means of thermal conversion of the dimethoxy-substituted solvent soluble phthalocyanine precursors
Fukuda, Takamitsu,Ishikawa, Naoto
, p. 151 - 154 (2014)
A simple procedure for the preparation o...
Improvement in the synthesis of metallophthalocyanines using microwave irradiation
Burczyk, Aleksandra,Loupy, André,Bogdal, Dariusz,Petit, Alain
, p. 179 - 188 (2005)
A successful application of microwave ir...
SYNTHESIS OF PHTHALOCYANINES FROM PHTHALONITRILE WITH ORGANIC STRONG BASES
Tomoda, Haruhiko,Saito, Shojiro,Ogawa, Shojiro,Shiraishi, Shinsaku
, p. 1277 - 1280 (1980)
In the presence of 1,8-diazabicycloundec...
Femtosecond nonlinear optical response of metallophthalocyanine films
Ma, Guohong,Guo, Lijun,Mi, Jun,Liu, Ye,Qian, Shixiong,Pan, Daocheng,Huang, Yue
, p. 633 - 638 (2001)
The third-order nonlinear optical proper...
-
Elvidge,Linstead
, p. 3536,3541 (1955)
-
Studies on polymorphic modifications of copper phthalocyanine
Achar,Lokesh
, p. 1987 - 1993 (2004)
Four α-, β-, γ- and ε-polymorphic forms ...
Effects of MN4-Type Coordination Structure in Metallophthalocyanine for Bio-Inspired Oxidative Desulfurization Performance
Tan, Amin,Tian, Min,Yang, Yan,Zhang, Gai,Zhang, Yufan
, (2022/02/14)
Oxidative desulfurization (ODS) is the p...
Sustainable approaches to the synthesis of metallophthalocyanines in solution
Imperatori, Patrizia,Paoletti, Anna Maria,Pennesi, Giovanna,Zanotti, Gloria
supporting information, (2021/06/15)
This work aims to investigate more susta...
Nanostructural catalyst: Metallophthalocyanine and carbon nano-onion with enhanced visible-light photocatalytic activity towards organic pollutants
Brzezinski, Krzysztof,Butsyk, Olena,Chaur, Manuel N.,Czyrko-Horczak, Justyna,Echegoyen, Luis,Olejnik, Piotr,Plonska-Brzezinska, Marta E.,Regulska, Elzbieta,Tomczykowa, Monika,Zubyk, Halyna
, p. 10910 - 10920 (2020/03/30)
Metallophthalocyanine (MPc) and carbon n...
Method for synthesizing metal phthalocyanine from phthalonitrile under catalysis of urea-choline chloride
-
Paragraph 0013, (2019/11/13)
The invention provides a clean method fo...
147-14-8 Process route
-
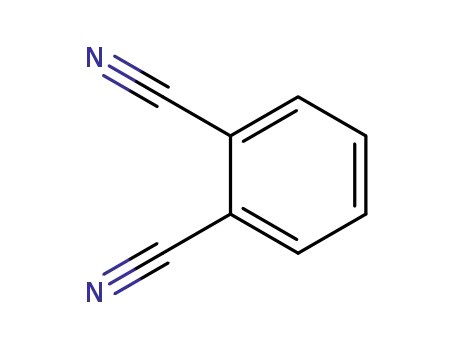
-
91-15-6
phthalonitrile

-
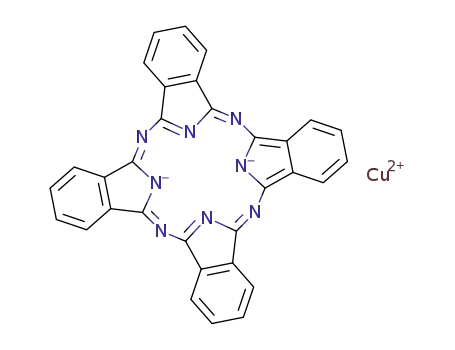
-
147-14-8
copper phthalocyanine
| Conditions | Yield |
|---|---|
|
With
CuCl2*2H2O;
for 0.166667h;
microwave irradiation;
|
98% |
|
With
CuCl2*2H2O;
at 190 - 210 ℃;
for 0.25h;
microwave irradiation;
|
88% |
|
With
copper diacetate; 1,8-diazabicyclo[5.4.0]undec-7-ene;
at 140 ℃;
for 0.0833333h;
|
76% |
|
With
1,1,1,3,3,3-hexamethyl-disilazane; copper(ll) bromide;
In
N,N-dimethyl-formamide;
at 100 ℃;
for 10h;
|
74% |
-
-
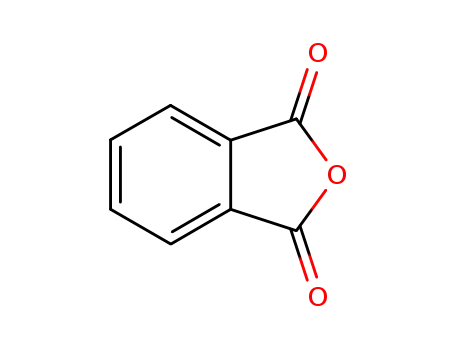
85-44-9
phthalic anhydride

-
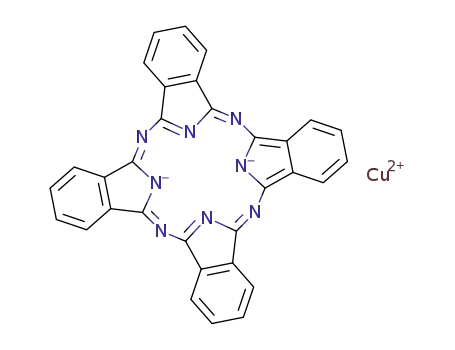
-
147-14-8
copper phthalocyanine
| Conditions | Yield |
|---|---|
|
With
ammonium molybdate tetrahydrate; urea;
for 0.0833333h;
microwave irradiation;
|
93% |
|
With
ammonium molybdate; urea;
at 200 ℃;
for 0.166667h;
microwave irradiation;
|
80% |
|
phthalic anhydride;
With
N,N-dimethyl-formamide; 1,1,1,3,3,3-hexamethyl-disilazane; copper dichloride;
toluene-4-sulfonic acid;
at 150 ℃;
for 10h;
With
sulfuric acid;
|
62% |
147-14-8 Upstream products
-
85-44-9

phthalic anhydride
-
91-15-6

phthalonitrile
-
136918-14-4
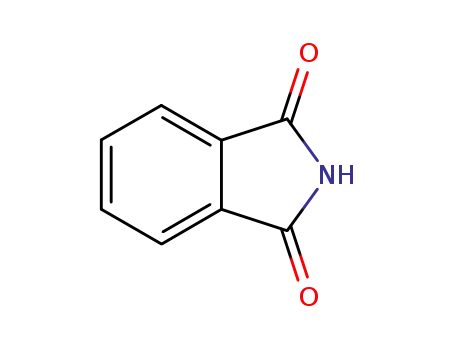
phthalimide
-
88-99-3
benzene-1,2-dicarboxylic acid
147-14-8 Downstream products
-
574-93-6
29H,31H-phthalocyanine
-
74-90-8

hydrogen cyanide
-
14832-14-5
C32Cl16CuN8, β
-
28802-09-7
copper(II) phthalocyaninetetrasulfonyl chloride
Relevant Products
-
Ipamorelin
CAS:170851-70-4
-
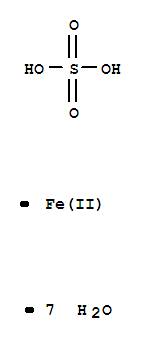
Ferrous sulfate heptahydrate
CAS:7782-63-0
-
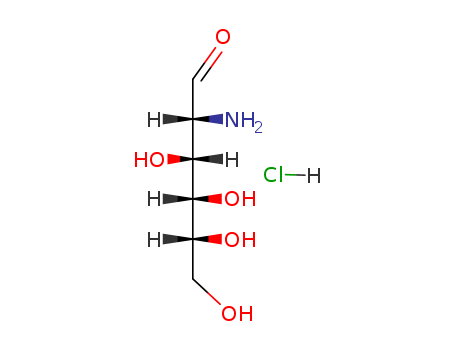
D-Glucosamine hydrochloride
CAS:66-84-2