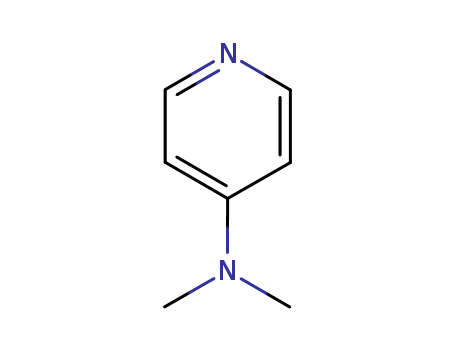
1122-58-3
- Product Name:4-Dimethylaminopyridine
- Molecular Formula:C7H10N2
- Purity:99%
- Molecular Weight:122.17
Product Details;
CasNo: 1122-58-3
Molecular Formula: C7H10N2
Appearance: white to yellow crystalline powder
Reputable supplier selling 4-Dimethylaminopyridine 1122-58-3 with stock
- Molecular Formula:C7H10N2
- Molecular Weight:122.17
- Appearance/Colour:white to yellow crystalline powder
- Vapor Pressure:0.431mmHg at 25°C
- Melting Point:83-86 °C(lit.)
- Refractive Index:n20/D 1.431
- Boiling Point:194.9 °C at 760 mmHg
- PKA:pKa (20°): 9.7
- Flash Point:71.7 °C
- PSA:16.13000
- Density:1.012 g/cm3
- LogP:1.14760
4-Dimethylaminopyridine(Cas 1122-58-3) Usage
|
Chemical Description |
4-dimethylaminopyridine is a catalyst that is commonly used in organic synthesis reactions. |
|
Reactions |
DMAP reacts readily with electrophilic reagents. It is possible to quaternize DMAP in high yield with either methyl iodide or ethyl bromide, decomposes quantitatively in the presence of aqueous alkali to N-methy1-4-pyridone[17]. Addition of DMAP to S, S'-diethyl-S, S'-dimethyl-S, S-1, 2vinylenedisulfonium salts results in the smooth formation of the salt with concomitant generation of ethyl methyl sulfide[19]. Reaction of DMAP with acetylenedicarboxylic acid leads spontaneously to the bis-adduct in high yields[20]. On reaction with perbenzoic acid the strongly polar N-oxide is formed. Nitration of DMAP with HNO3/H2SO4 gives the 3-nitro derivatives in 81 % yield and, under forcing condition; the 3,5-dinitro compounds are obtained[16]. Reaction with O-(p-toluenesulfony1)hydroxylamine affords the N-amino compound in 67% yield which is isolated as the perchlorate. By treatment with D2O it is possible to selectively exchange the a-protons in DMAP, with DClO4 to exchange the P-protons to furnish and with D2O/NaOD to replace all aromatic protons by deuterium[18]. |
|
Application as acylation catalysts |
Acylation of alcohol The high catalytic activity of DMAP and PPY can be used for acylating sterically hindered secondary or tertiary alcohols with carboxylic anhydrides or acyl halides when the pyridine method fails. In most cases, it is necessary to use only 0.05-0.2 mol of catalyst per mol of substance and the acid that is formed can be bound with an equivalent amount of trimethylamine[21, 22] or pyridine[20]. Such solvents as hexane, toluene, benzene, methylene chloride, chloroform, ethyl acetate, tetrahydrofuran, triethylamine, pyridine, or acetic anhydride are suitable for use with these catalysts. Among the tertiary alcohols which can be easily acylated with DMAP and PPY, mention should be made of l-methyl cyclohexanol, 1-ethynylcyclohexanol, 1,l-diphenylethanol, linalool, l, l-dimethoxy-2-methyl-3-buten-2-ol, 5,5-dimethoxy-2-methyI-3-pentyn-2-ol, and cis-4- (1-hydroxyisopropyl)-2-methylcyclohexanone. Acylation of phenols In the acylation of phenols, DMAP and PPY effect a similar increase in reaction rate as is found in the case of alcohols. Hence, the method is of interest for the acylation of sterically hindered phenols. For example, mesitol can be smoothly acetylated with acetic anhydride/DMAP to 2,5-ditert-butylphenol and analogous compounds can be transformed into acyl derivatives of the type in high yields[24]. 11,12-Dihydroglaziovine smoothly affords the acyl derivative[23, 25]. Acylation of amines DMAP and PPY have been seldom used for the acylation of amines. The kinetic investigations of Lituinenko and Kirichenko [26] have shown that an enormous increase in reaction rate is observed when acylations are carried out in aprotic solvents. These authors have determined the following relative rate constants (in parentheses) for the amine-catalyzed acylation of m-chloroaniline with benzoyl chloride in benzene: N, N-dimethylaniline (0.1); triethylamine (0.072); 2,6-dimethylpyridine (0.03); pyridine (1.80); 4-methylpyridine (10.0); and DMAP (10600). Acylation of enolates Acylations involving CH-acid compounds which can be performed with pyridine or triethylamine as catalyst are found to proceed at a much higher rate when DMAP or PPY is used. The Dakin-West reaction of N-acyl amino acids, in which a 2-oxazolin-5-one is acetylated at C-4 with a carboxylic anhydride in pyridine with formation of a new C-C bond, has been extensively investigated[27]. The combination products, consisting of the ambident oxazolin-5-one anions and N-acylpyridinium cations initially formed under kinetic control, are transformed via the ion pair into the thermodynamically most stable product[28]. Decarboxylative ring opening by the subsequently formed carboxylic acid yields the a-acyl amino ketone[29, 30]. Reactions of isocyanates Pyridine-catalyzed reactions of isocyanates with carboxylic acids to form amides are found to be strongly accelerated on replacement of pyridine by DMAP. Phenylacetic acid is found to react with phenyl isocyanate in 1,2-dichloroethane at 24°C to give the amide in 66 % yield in less than 5 min; whereas on using the same amount of pyridine only 53% could be isolated after 2h. With triethylamine, only very little is formed besides diphenylurea[31]. Miscellaneous Applications DMAP has been used in the hardening of epoxy resins with dicyanodiamine, in the transformation of nitriles into thionamides, and in the transfer of silyl groups to tertiary hydroxyl groups[32, 33]. Transfer of Functional Groups Dimethylarninopyridinium salts are interesting reagents for the transfer of acyl and also cyano and phosphono groups in aqueous medium[34, 35]. |
|
Flammability and Explosibility |
Nonflammable |
|
Purification Methods |
Recrystallise DMAP from toluene [Sadownik et al. J Am Chem Soc 108 7789 1986]. [Beilstein 22 V 112.] § A polystyrene supported version (PS-DMAP) is commercially available. |
|
Redox Properties |
Modifications to the molecular skeleton of neutral bis-2-(4-dimethylamino)pyridinylidene electron donors, derived from 4DMAP, have been explored. |
|
Pharmaceutical Applications |
Cocrystals/salt formations are studied for modulating the solubility, stability, and hygroscopicity of pharmaceutical ingredients. 4DMAP, with its ability to accept H-transfer, forms salts with organic acids, leading to complex non-covalent bonds. |
|
Supramolecular Crystal Engineering |
Organic acids with suitable donor and acceptor properties play a crucial role in multi-component assembly, forming a dimer, caterer, and bridged synthons in the solid state. The interaction between organic acids and 4DMAP, with its subsidiary N(CH3)2, results in complex non-covalent bonds, facilitating supramolecular crystal engineering. |
|
Overview |
4-Dimethylaminopyridine is highly powerful catalyst of organic synthesis. The treatment of substrates such as alcohols, phenols and amines with acetic anhydride (or acetyl chloride) in the presence of pyridine has provided a general acetylation method since the turn of the 20th century. However, this approach often proves to be unsatisfactory for the acetylation of deactivated substrates. It was not until the late 1960’s that certain 4-dialkylaminopyridines were found (independently by two research groups)[1, 2] to be much superior to pyridine as catalysts for difficult acetylations or acylations, in general. Figure 1 the chemical structure of DMAP 4-Dialkylaminopyridines were soon found to have general applicability for catalysis of a wide variety of reactions. 4-dimethylaminopyridine’s (DMAP) wide applicability has been frequently reviewed since the first review appeared in 1978. [3] The accelerating pace of reported applications for DMAP and the availability of DMAP in commercial quantities, at modest prices, have continued to stimulate great interest in its use as a catalyst in the fields of organic, polymer, analytical and biochemistry. Today there are thousands of examples of the use of DMAP in far ranging fields of chemistry in both patents and the research literature. Many full-scale production processes utilizing DMAP have been and are being operated. Several pharmaceutical and agricultural products that rely on DMAP’s superior catalytic properties in their synthetic sequences have been produced for years. Since 1976 more than 11,000 US patents have been granted which mention DMAP or dimethylaminopyridine. The functional groups and class of compounds that are involved in the reactions with DMAP include alcohols, amines, arenes, azides, carbenes, enols, epoxides, hydrazines, hydroxylamines, phenols, thiols, lipids, sugars, aminoacids, peptides, alkaloids, steroids, terpenes, and others. Reactions that have been published in the literature using DMAP fall into, but are not limited to, the following types of reactions: Acylation; Acetylation; Alkylation; Benzoylation; Bischler-Naperalski cyclization, Carbonylation; Carbo-diimidation; Cyclization; Dehydration; Esterificaton; Indole Synthesis; Nucleophilic Substitution; Rearrangement; Silylation; Sulfonamidation; Sulfonation; Tritylation; Formylation; Carbamoylation; Phosphorylation; Lactonization; Pivaloylation; Dakin-West Reaction; Baylis-Hillman Reaction. |
|
General Description |
Valacyclovir Related Compound G, also called as 4-(Dimethylamino)pyridine (DMAP) is an excellent catalyst for acylation of hindered alcohols and in chemical transformations. It is highly nucleophilic in nature. |
InChI:InChI=1/C7H10N2/c1-9(2)7-3-5-8-6-4-7/h3-6H,1-2H3/p+1
1122-58-3 Relevant articles
Models for B12-conjugated radiopharmaceuticals. Cobaloxime binding to new fac-[Re(CO)3(Me2bipyridine)(amidine)]BF4 complexes having an exposed pyridyl nitrogen
Lewis, Nerissa A.,Marzilli, Patricia A.,Fronczek, Frank R.,Marzilli, Luigi G.
, p. 11096 - 11107 (2014)
New mononuclear amidine complexes, fac-[...
-
Su,Watson
, p. 1854 (1974)
-
N-Heteroarylphosphonates, Part II. Synthesis and reactions of 2- and 4- phosphonatoquinolines and related compounds
Haase, Mirko,Guì?nther, Wolfgang,Goì?rls, Helmar,Anders, Ernst
, p. 2071 - 2081 (1999)
We extend our synthetic method for the e...
Continuous flow nucleophilic aromatic substitution with dimethylamine generated in situ by decomposition of DMF
Petersen, Trine P.,Larsen, Anders Foller,Ritzen, Andreas,Ulven, Trond
, p. 4190 - 4195 (2013)
A safe, practical, and scalable continuo...
Synthesis of aminopyridines via an unprecedented nucleophilic aromatic substitution of cyanopyridines
Penney, Jonathan M.
, p. 2667 - 2669 (2004)
The direct reaction of 2- and 4-cyanopyr...
A Novel One-Pot Synthesis of N,N-Dimethylaminopyridines by Diazotization of Aminopyridines in Dimethylformamide in the Presence of Trifluoromethanesulfonic Acid
Filimonov, V. D.,Krasnokutskaya, E. A.,Potapova, M. I.,Sanzhiev, A. N.
, p. 1023 - 1028 (2020)
Abstract: Diazotization of aminopyridine...
A simple synthesis of aminopyridines: Use of amides as amine source
Kodimuthali, Arumugam,Mungara, Anitha,Prasunamba, Padala Lakshmi,Pal, Manojit
, p. 1439 - 1445 (2010)
A transition metal/microwave irradiation...
-
Anderson,Seeger
, p. 340 (1949)
-
-
Jameson,Lawlov
, p. 53,55 (1970)
-
Mechanistic insight gained into the ligand substitution reactions of bis(o-benzosemiquinonediiminato)(triphenylphosphane)cobalt(III) - Kinetic, solvent, and volume profile studies
Alzoubi, Basam M.,Hamza, Mohamed S. A.,Duecker-Benfer, Carlos,Van Eldik, Rudi
, p. 2972 - 2978 (2003)
The ligand substitution reactions of bis...
Formation of Singlet Oxygen in the Deoxygenation of Heteroarene N-Oxides by Dimethyldioxirane
Adam, Waldemar,Briviba, Karlis,Duschek, Frank,Golsch, Dieter,Kiefer, Wolfgang,Sies, Helmut
, p. 1831 - 1832 (1995)
4-Dimethylaminopyridine-N-oxide 2 and 2'...
-
Wompe et al.
, p. 361 (1958)
-
A Lewis Base Nucleofugality Parameter, NFB, and Its Application in an Analysis of MIDA-Boronate Hydrolysis Kinetics
García-Domínguez, Andrés,Gonzalez, Jorge A.,Leach, Andrew G.,Lloyd-Jones, Guy C.,Nichol, Gary S.,Taylor, Nicholas P.
supporting information, (2022/01/04)
The kinetics of quinuclidine displacemen...
Boron-Containing Organic Diradicaloids: Dynamically Modulating Singlet Diradical Character by Lewis Acid-Base Coordination
Dou, Chuandong,Guo, Jiaxiang,Wang, Yue,Yang, Yue
supporting information, p. 18272 - 18279 (2021/11/12)
Organic diradicaloids have unique open-s...
Simple RuCl3-catalyzed N-Methylation of Amines and Transfer Hydrogenation of Nitroarenes using Methanol
Sarki, Naina,Goyal, Vishakha,Tyagi, Nitin Kumar,Puttaswamy,Narani, Anand,Ray, Anjan,Natte, Kishore
, p. 1722 - 1729 (2021/04/19)
Methanol is a potential hydrogen source ...
Lewis Acidic Boranes, Lewis Bases, and Equilibrium Constants: A Reliable Scaffold for a Quantitative Lewis Acidity/Basicity Scale
Mayer, Robert J.,Hampel, Nathalie,Ofial, Armin R.
supporting information, p. 4070 - 4080 (2021/01/29)
A quantitative Lewis acidity/basicity sc...
1122-58-3 Process route
-
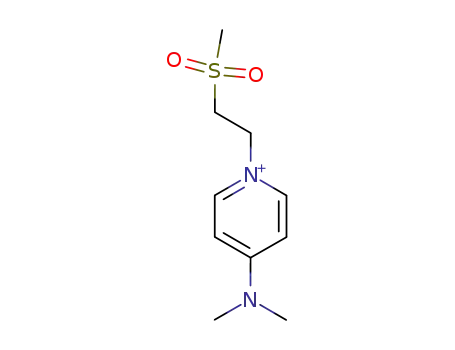
-
141375-40-8
4-Dimethylamino-1-(2-methanesulfonyl-ethyl)-pyridinium

-
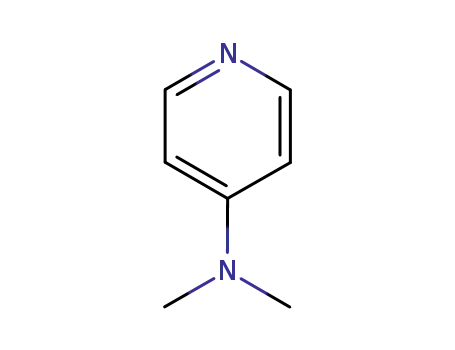
-
1122-58-3
dmap

-
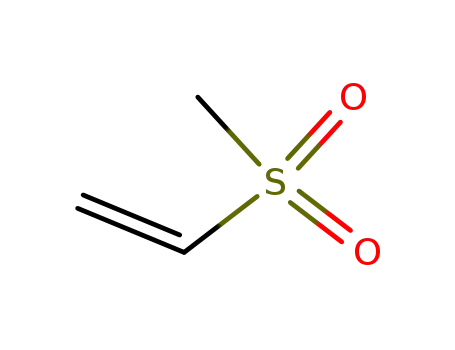
-
3680-02-2
methyl ethenyl sulphone
| Conditions | Yield |
|---|---|
|
With
water;
hydroxide;
at 25 ℃;
Equilibrium constant;
Mechanism;
|
-
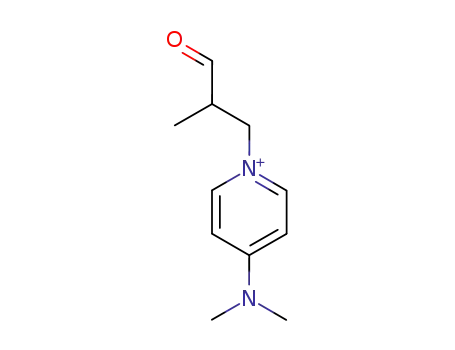
-
141375-45-3
2-methyl-3-(4-(dimethylamino)pyridinium)propanal

-

-
1122-58-3
dmap

-
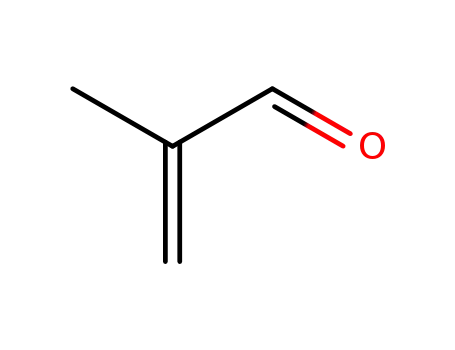
-
78-85-3,25120-30-3
2-methylpropenal
| Conditions | Yield |
|---|---|
|
With
water;
hydroxide;
at 25 ℃;
Equilibrium constant;
Mechanism;
|
1122-58-3 Upstream products
-
504-24-5
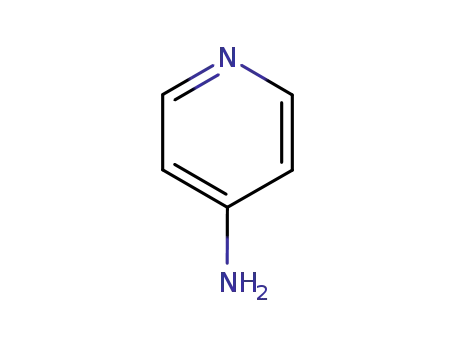
4-aminopyridine
-
67-56-1
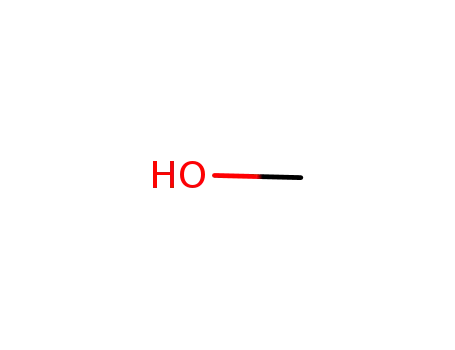
methanol
-
4783-86-2
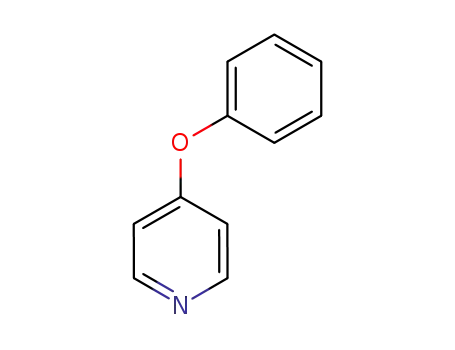
4-phenoxypyridine
-
506-59-2
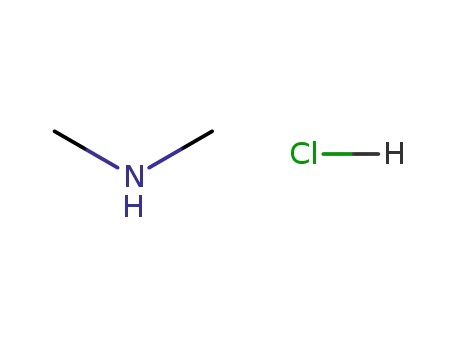
N,N-dimethylammonium chloride
1122-58-3 Downstream products
-
86422-09-5
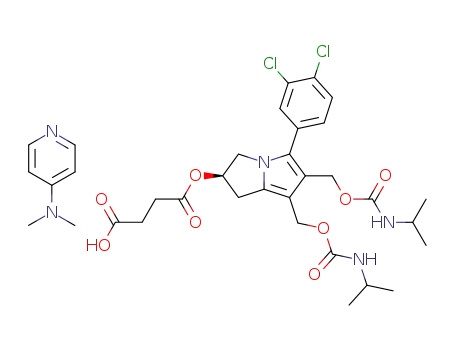
4-(dimethylamino)pyridinium 5-(3,4-dichlorophenyl)-6,7-bis(hydroxymethyl)-2-<(carboxylatoethyl)carbonyl>-2,3-dihydro-1H-pyrrolizine bis(2-propylcarbamate)
-
95115-18-7
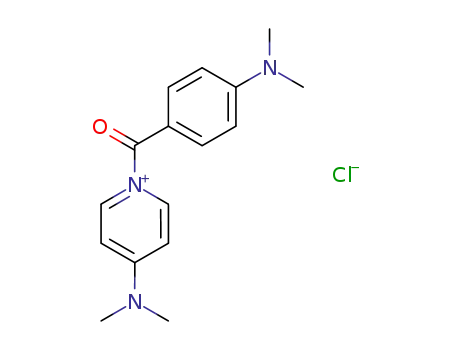
N-(4-dimethylaminobenzoyl)-4-dimethylaminopyridinium chloride
-
111055-10-8
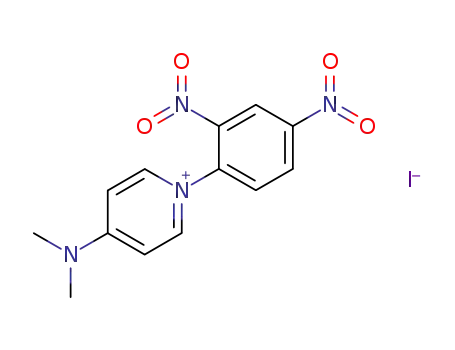
4-Dimethylamino-1-(2,4-dinitro-phenyl)-pyridinium; iodide
-
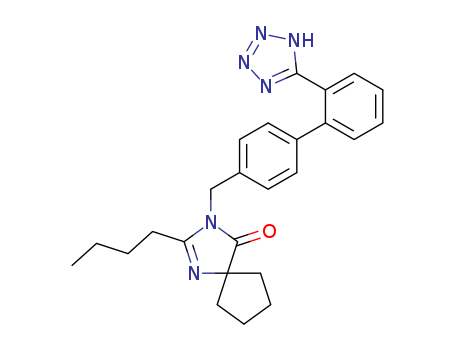
134723-99-2
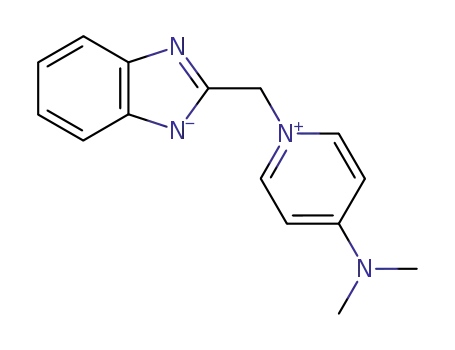
C15H16N4
Relevant Products
-
Tesamorelin
CAS:218949-48-5
-
Irbesartan
CAS:138402-11-6
-
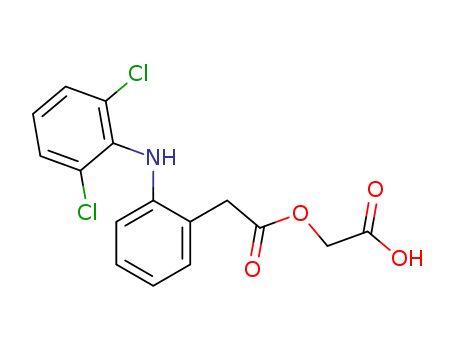
Aceclofenac
CAS:89796-99-6