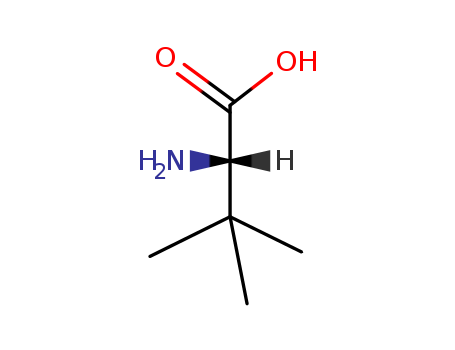
20859-02-3
- Product Name:L-tert-Leucine
- Molecular Formula:C6H13NO2
- Purity:99%
- Molecular Weight:131.175
Product Details;
CasNo: 20859-02-3
Molecular Formula: C6H13NO2
Appearance: white to almost white powder
Chemical plants supply high-quality L-tert-Leucine 20859-02-3 in bulk
- Molecular Formula:C6H13NO2
- Molecular Weight:131.175
- Appearance/Colour:white to almost white powder
- Vapor Pressure:0.0499mmHg at 25°C
- Melting Point:300 °C
- Refractive Index:-9 ° (C=3, H2O)
- Boiling Point:217.7 °C at 760 mmHg
- PKA:2.39±0.12(Predicted)
- Flash Point:85.5 °C
- PSA:63.32000
- Density:1.038 g/cm3
- LogP:1.14470
L-tert-Leucine(Cas 20859-02-3) Usage
|
Synthesis Reference(s) |
Organic Syntheses, Coll. Vol. 3, p. 523, 1955Tetrahedron Letters, 19, p. 4625, 1978 DOI: 10.1016/S0040-4039(01)85688-4 |
InChI:InChI=1/C6H13NO2/c1-6(2,3)4(7)5(8)9/h4H,7H2,1-3H3,(H,8,9)
20859-02-3 Relevant articles
Asymmetric Strecker synthesis using enantiopure sulfinimines and diethylaluminum cyanide: The alcohol effect
Davis, Franklin A.,Portonovo, Padma S.,Reddy, Rajarathnam E.,Chiu, Yu-Hung
, p. 440 - 441 (1996)
-
Molecular Structure of a Chiral 3,5-Bridged Pyridine and the Effect of Structure on Circular Dichroic Spectra
Speelman, Johanna C.,Talma, Auke G.,Kellogg, Richard M.,Meetsma, A.,Boer, J. L. de,et al.
, p. 1055 - 1062 (1989)
The crystal structure of the 3,5-bridged...
Design of a self-sufficient hydride-shuttling cascade for concurrent bioproduction of 7,12-dioxolithocholate andl-tert-leucine
Chen, Qi,Han, Yu,Li, Chun-Xiu,Pan, Jiang,Qian, Xiao-Long,Xu, Jian-He,Yang, Bing-Yi,You, Zhi-Neng,Zhou, Ke
, p. 4125 - 4133 (2021)
Oxidoreductase-mediated biotransformatio...
-
Tanabe,T. et al.
, p. 2178 - 2179 (1968)
-
SIMPLE OPTICAL RESOLUTION OF TERLEUCINE
Viret, Joelle,Patzelt, Heiko,Collet, Andre
, p. 5865 - 5868 (1986)
Underivatized terleucine (1) can be conv...
Asymmetric synthesis of l-6-hydroxynorleucine from 2-keto-6-hydroxyhexanoic acid using a branched-chain aminotransferase
Seo, Young-Man,Kim, Aran,Bea, Han-Seop,Lee, Sang-Hyeup,Yun, Hyungdon
, p. 171 - 176 (2012)
l-6-Hydroxynorleucine was synthesized fr...
Cadmium sulfide net framework nanoparticles for photo-catalyzed cell redox
Chang, Zhaoyu,Dong, Wanyuan,Meng, Xiangqi,Ren, Yuhong,Wang, Hualei,Wei, Dongzhi,Zhang, Jian
, p. 37820 - 37825 (2020)
A strategy for synthesizing cadmium sulf...
Biocatalytic cascade reactions for asymmetric synthesis of aliphatic amino acids in a biphasic reaction system
Park, Eul-Soo,Shin, Jong-Shik
, p. 9 - 14 (2015)
Abstract Enantiopure aliphatic amino aci...
Formate Dehydrogenase from Rhodococcus jostii (RjFDH) – A High-Performance Tool for NADH Regeneration
Boldt, Alexander,Ansorge-Schumacher, Marion B.
, p. 4109 - 4118 (2020)
The use of formate dehydrogenases (FDHs)...
Artificial multienzyme supramolecular device: Highly ordered self-assembly of oligomeric enzymes in vitro and in vivo
Gao, Xin,Yang, Shuai,Zhao, Chengcheng,Ren, Yuhong,Wei, Dongzhi
, p. 14027 - 14030 (2014)
A strategy for scaffold-free self-assemb...
A Transient Directing Group Strategy Enables Enantioselective Multicomponent Organofluorine Synthesis
Engle, Keary M.,Gao, Yang,Li, Zi-Qi,Liu, Mingyu,Liu, Zhonglin,Oxtoby, Lucas J.,Tran, Van T.
supporting information, p. 8962 - 8969 (2021/07/01)
The vicinal fluorofunctionalization of a...
Synthesis method of L-tertiary leucine and L-cyclohexyl alanine
-
Paragraph 0030-0032, (2021/08/07)
The invention discloses a synthesis meth...
Method for preparing D-type or L-type tert-leucine
-
Paragraph 0036-0039, (2020/06/16)
The invention discloses a method for pre...
20859-02-3 Process route
-
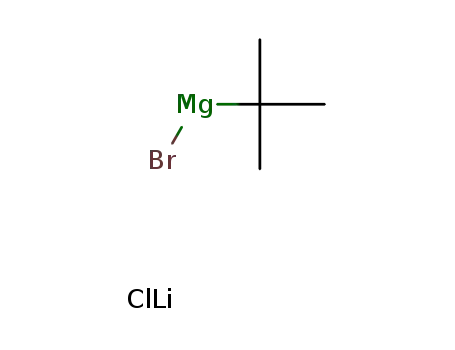
-
tert-butylmagnesium bromide-lithium chloride

-
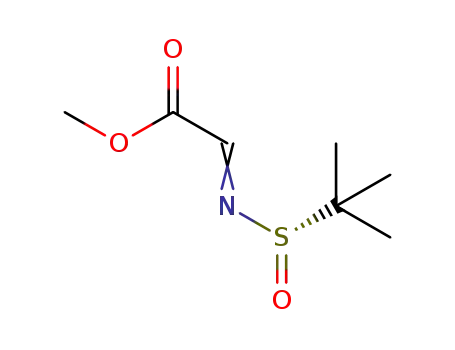
-
(S)-(2-methylpropane-2-sulfinimido)-acetate methyl ester

-
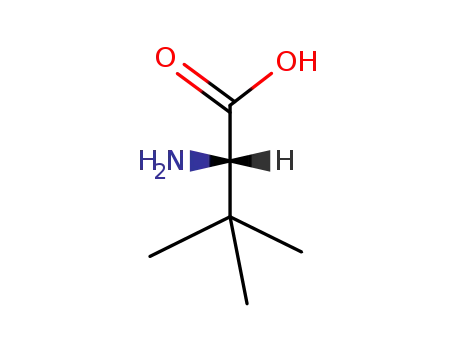
-
20859-02-3
L-tert-Leucine

-
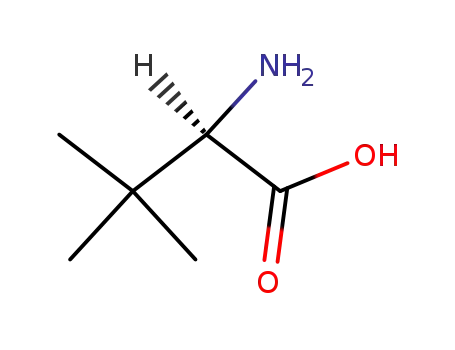
-
26782-71-8
D-tert-leucine
| Conditions | Yield |
|---|---|
|
tert-butylmagnesium bromide-lithium chloride; (S)-(2-methylpropane-2-sulfinimido)-acetate methyl ester;
In
tetrahydrofuran; dichloromethane;
at -20 - 0 ℃;
for 3h;
Inert atmosphere;
With
hydrogenchloride;
In
ethyl acetate;
at 30 - 35 ℃;
With
water; lithium hydroxide;
In
tetrahydrofuran;
at 25 - 30 ℃;
for 2h;
Overall yield = 84.2 percent;
|
88.2 % ee |
-
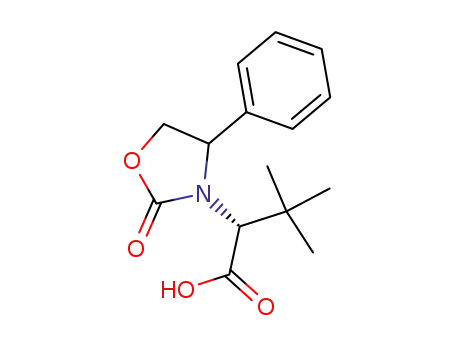
-
(R)-3,3-Dimethyl-2-(2-oxo-4-phenyl-oxazolidin-3-yl)-butyric acid

-

-
20859-02-3
L-tert-Leucine

-

-
26782-71-8
D-tert-leucine
| Conditions | Yield |
|---|---|
|
With
ammonia; lithium;
In
tetrahydrofuran; tert-butyl alcohol;
at -78 ℃;
for 0.5h;
Yield given. Title compound not separated from byproducts;
|
20859-02-3 Upstream products
-
62965-57-5
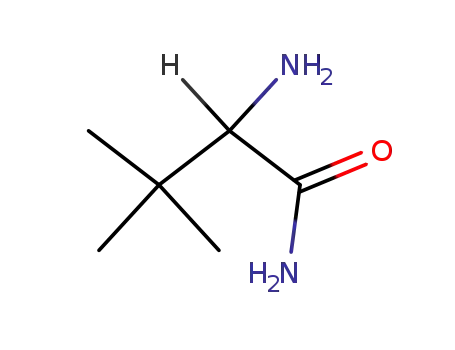
(±)-2-amino-3,3-dimethylbutanamide
-
92571-61-4
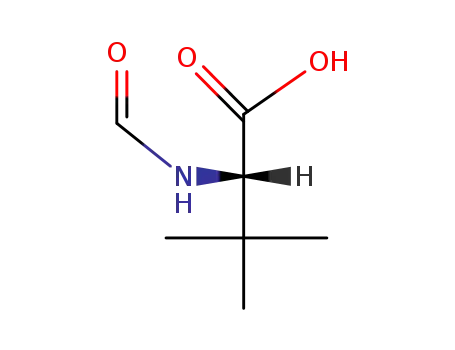
L-2-formylamino-3,3-dimethyl-butyric acid
-
62965-10-0
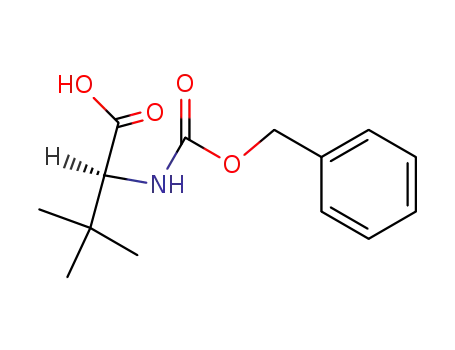
(S)-N-carbobenzoxy-tert-butylleucine
-
3850-31-5
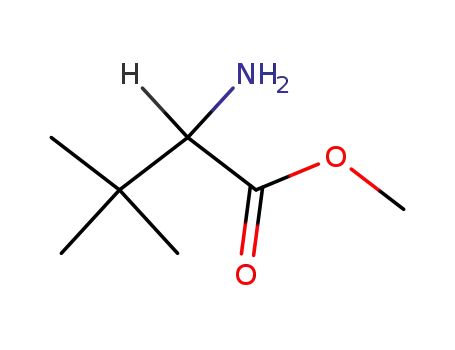
methyl tert-leucinate
20859-02-3 Downstream products
-
62965-10-0

(S)-N-carbobenzoxy-tert-butylleucine
-
63038-26-6
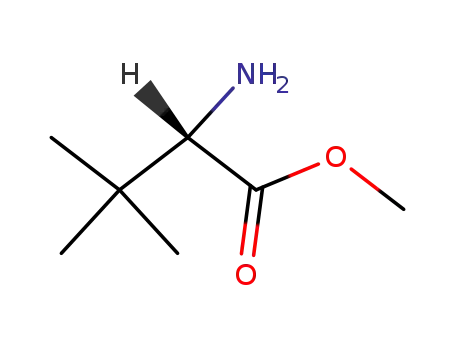
(S)-2-amino-3,3-dimethyl-butyric acid methyl ester
-
62965-35-9
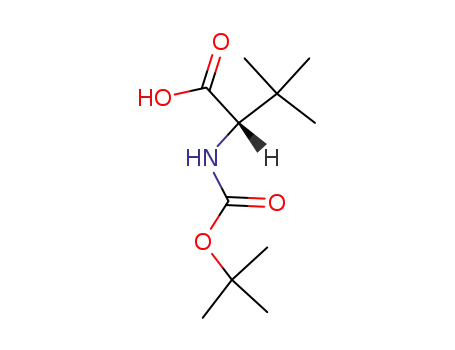
N-tert-butyloxycarbonyl-L-tert-leucine
-
31556-73-7
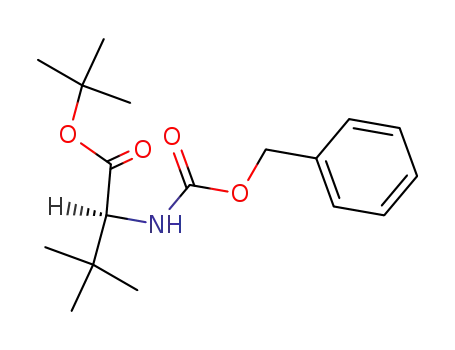
Z-L-Tle-OtBu
Relevant Products
-
Tesamorelin
CAS:218949-48-5
-
Lovastatin
CAS:75330-75-5
-
Irbesartan
CAS:138402-11-6