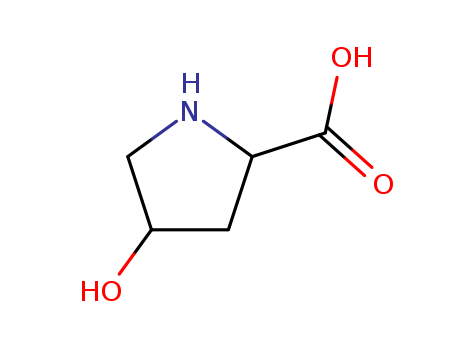
51-35-4
- Product Name:L-Hydroxyproline
- Molecular Formula:C5H9NO3
- Purity:99%
- Molecular Weight:131.131
Product Details;
CasNo: 51-35-4
Molecular Formula: C5H9NO3
Appearance: white crystalline powder
Factory supply good quality L-Hydroxyproline 51-35-4 with stock
- Molecular Formula:C5H9NO3
- Molecular Weight:131.131
- Appearance/Colour:white crystalline powder
- Melting Point:273 °C (dec.)(lit.)
- Refractive Index:-75.5 ° (C=4, H2O)
- Boiling Point:355.2 °C at 760 mmHg
- PKA:1.82, 9.66(at 25℃)
- Flash Point:168.6 °C
- PSA:69.56000
- Density:1.395 g/cm3
- LogP:-0.87740
L-Hydroxyproline(Cas 51-35-4) Usage
|
Production Methods |
In the past L-Hydroxyproline was isolated by hydrolysis of animal collagen, e. g. gelatine or collagen, of which it is a major constituent. L-Hydroxyproline is an unnatural amino acid which is made in the body by hydroxylation of L-Proline. The presence of L-Hydroxyproline and proline are key to maintaining the stability of the tight collagen helix. Today L-Hydroxyproline is manufactured by bio-catalysed hydroxylation of proline in bacteria. Together with its other isomers, L-Hydroxyproline is also used as an inter mediate for a range of pharmaceutical active ingredients. Produced by hydroxylation of L-proline after protein synthesis. It is contained rich in collagen and elastin. Known to stabilization of triple-helix structure of collagen. Widely used as an intermediate for medicines. |
|
Biochem/physiol Actions |
Trans-4-Hydroxy-L-proline is used in the organic synthesis of depsilairdin analogues, noncompetitive peptidomimetic inhibitors of multidrug resistance P-glycoprotein and 3-guaninyl-5-hydroxymethyl-2-pyrrolidinone (4) or 3-adeninyl-5-hydroxymethyl-2-pyrrolidinone (5) nucleoside analogues. |
|
Purification Methods |
Crystallise it from MeOH/EtOH (1:1). Separation from normal allo-isomer can be achieved by crystallisation of the copper salts [see Levine Biochemical Preparations 8 114 1961]. Separation from proline is achieved via the crystalline picrate, CdCl2, or acid ammonium rhodanate salts [see Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 p 2182 1961, Kapfhammer & Mohn Z Physiol Chem 306 76 1956]. [Beilstein 22/5 V 7.] |
|
Role in Collagen Synthesis |
Hydroxyproline is a non-proteinogenic amino acid formed by the hydroxylation of proline. It plays a pivotal role in stabilizing the helical structure of collagen fibers, contributing to the structural integrity of connective tissues. |
|
Role in Disease Pathophysiology |
Abnormalities in hydroxyproline metabolism are implicated in various diseases, including graft versus host disease, keloids, and vitiligo. Elevated levels of hydroxyproline are observed in these disorders, while decreased levels indicate poor wound healing. |
|
Significance in Liver Health |
In liver fibrosis, hydroxyproline levels in tissues, serum, and urine serve as important indicators of fibrogenesis rates and disease progression. Its presence in the extracellular matrix (ECM) produced by hepatic stellate cells (HSCs) helps preserve liver cell integrity and function. |
|
Diagnostic Marker |
Hydroxyproline estimation is utilized as a marker for diagnosing liver fibrosis and evaluating the anti-fibrotic effects of therapeutic interventions, including herbal and non-herbal medicines. It serves as a noninvasive biomarker for detecting severe fibrosis in chronic liver diseases. |
|
Biochemical Properties |
Hydroxyproline, along with proline, constitutes about one-third of the amino acids in collagen synthesis. |
|
Enzymatic Hydroxylation |
Hydroxyproline formation involves the catalytic hydroxylation of proline units by proline hydroxylase enzyme, utilizing ascorbic acid and oxygen as cofactors. |
|
Definition |
ChEBI: An optically active form of 4-hydroxyproline having L-trans-configuration. |
|
General Description |
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards |
InChI:InChI:1S/C5H9NO3/c7-3-1-4(5(8)9)6-2-3/h3-4,6-7H,1-2H2,(H,8,9)
51-35-4 Relevant articles
Recharacterization of the mammalian cytosolic type 2 (R)-β-hydroxybutyrate dehydrogenase as 4-oxo-L-proline reductase (EC 1.1.1.104)
Bozko, Maria,Drozak, Jakub,Jagielski, Adam K.,Kocdemir, Kubra,Kwiatkowski, Sebastian,Witecka, Apolonia,Zarod, Michal
, (2022/03/23)
Early studies revealed that chicken embr...
Discovery of New Fe(II)/α-Ketoglutarate-Dependent Dioxygenases for Oxidation of l-Proline
Dussauge, Solene,Moore, Charles,Snajdrova, Radka,Tassano, Erika,Vargas, Alexandra
supporting information, (2022/02/09)
Genome mining for novel Fe(II)/α-ketoglu...
Studies on the selectivity of proline hydroxylases reveal new substrates including bicycles
Smart, Tristan J.,Hamed, Refaat B.,Claridge, Timothy D.W.,Schofield, Christopher J.
supporting information, (2019/11/26)
Studies on the substrate selectivity of ...
Discovery of new A- and B-type laxaphycins with synergistic anticancer activity
Cai, Weijing,Matthew, Susan,Chen, Qi-Yin,Paul, Valerie J.,Luesch, Hendrik
, p. 2310 - 2319 (2018/04/02)
Two new cyclic lipopeptides termed laxap...
51-35-4 Process route
-
type I collagen from Bester sturgeon scales
-
type I collagen from Bester sturgeon scales

-
-
56-41-7,25191-17-7,18875-37-1
L-alanin

-
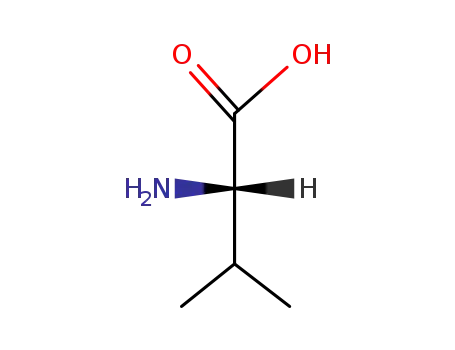
-
72-18-4,25609-85-2,7004-03-7,921-10-8
L-valine

-
-
56-45-1,83245-15-2
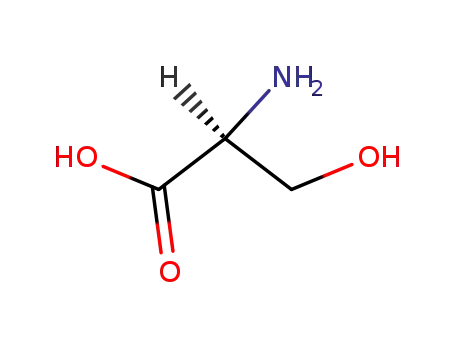
L-serin

-
-
72-19-5,134357-96-3,7013-32-3,82822-12-6
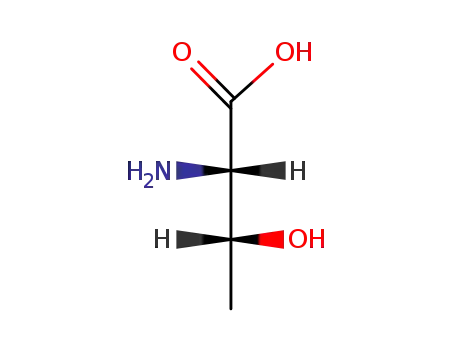
L-threonine

-
-
61-90-5,21675-61-6,25248-98-0,70-45-1
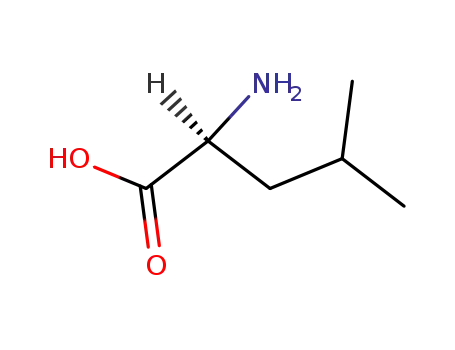
L-leucine

-
-
73-32-5,959215-79-3,18875-42-8
L-isoleucine

-
-
63-68-3,26062-47-5,58576-49-1
L-methionine

-
-
56-87-1,3506-25-0
L-lysine

-
-
56-84-8,25608-40-6,27881-03-4,32505-46-7,52526-39-3
L-Aspartic acid

-
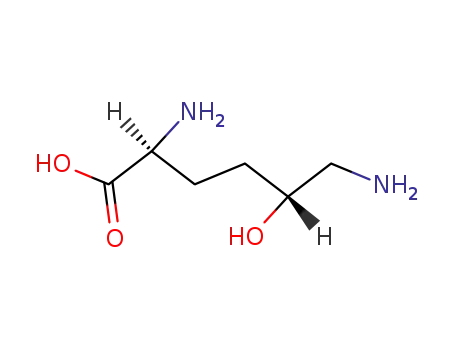
-
504-91-6,1190-94-9,3506-26-1,6000-08-4,18899-29-1,18899-30-4,18899-31-5,52153-41-0,78088-29-6,28902-93-4
(5R)-5-hydroxy-L-lysine

-
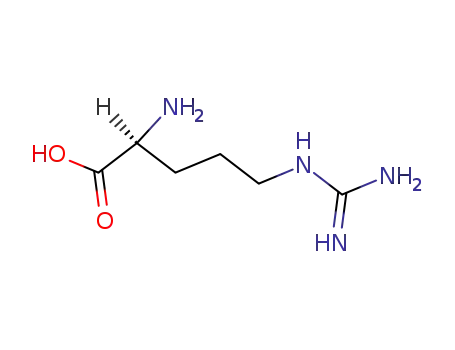
-
74-79-3,25212-18-4
L-arginine

-
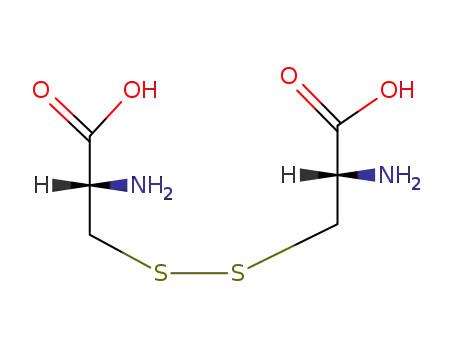
-
349-46-2
S,S-cystine

-
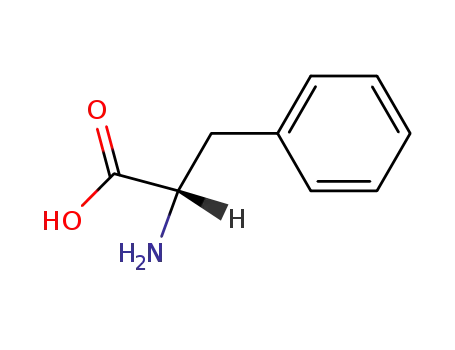
-
63-91-2,25191-15-5,658-69-5,67675-33-6
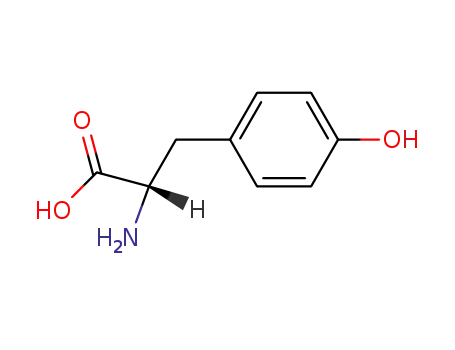
L-phenylalanine

-
-
60-18-4,18875-48-4,25619-78-7,30704-25-7
L-tyrosine

-
-
56-40-6,18875-39-3,25718-94-9
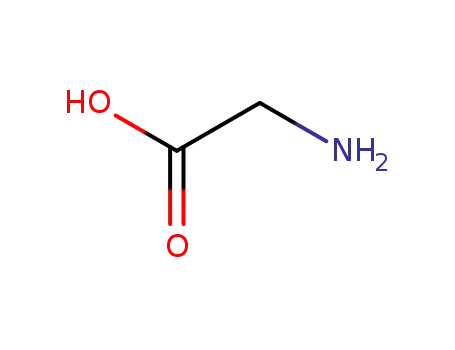
glycine

-
-
147-85-3,25191-13-3,37159-97-0,4305-67-3,4607-28-7
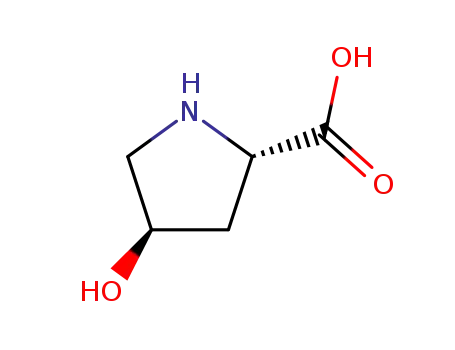
L-proline

-
-
51-35-4,30724-02-8
4R-4-hydroxyproline

-
-
71-00-1
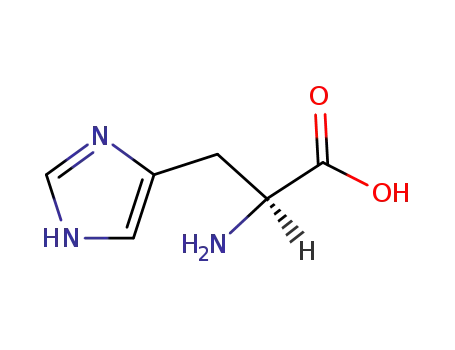
L-histidine
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; water;
at 110 ℃;
for 24h;
|
-
type I collagen from Bester sturgeon skin
-
type I collagen from Bester sturgeon skin

-

-
56-41-7,25191-17-7,18875-37-1
L-alanin

-

-
72-18-4,25609-85-2,7004-03-7,921-10-8
L-valine

-

-
56-45-1,83245-15-2
L-serin

-

-
72-19-5,134357-96-3,7013-32-3,82822-12-6
L-threonine

-

-
61-90-5,21675-61-6,25248-98-0,70-45-1
L-leucine

-

-
73-32-5,959215-79-3,18875-42-8
L-isoleucine

-

-
63-68-3,26062-47-5,58576-49-1
L-methionine

-

-
56-87-1,3506-25-0
L-lysine

-

-
56-84-8,25608-40-6,27881-03-4,32505-46-7,52526-39-3
L-Aspartic acid

-

-
504-91-6,1190-94-9,3506-26-1,6000-08-4,18899-29-1,18899-30-4,18899-31-5,52153-41-0,78088-29-6,28902-93-4
(5R)-5-hydroxy-L-lysine

-

-
74-79-3,25212-18-4
L-arginine

-

-
349-46-2
S,S-cystine

-

-
63-91-2,25191-15-5,658-69-5,67675-33-6
L-phenylalanine

-

-
60-18-4,18875-48-4,25619-78-7,30704-25-7
L-tyrosine

-

-
56-40-6,18875-39-3,25718-94-9
glycine

-
-
147-85-3,25191-13-3,37159-97-0,4305-67-3,4607-28-7
L-proline

-

-
51-35-4,30724-02-8
4R-4-hydroxyproline

-

-
71-00-1
L-histidine
| Conditions | Yield |
|---|---|
|
With
hydrogenchloride; water;
at 110 ℃;
for 24h;
|
51-35-4 Upstream products
-
217184-95-7
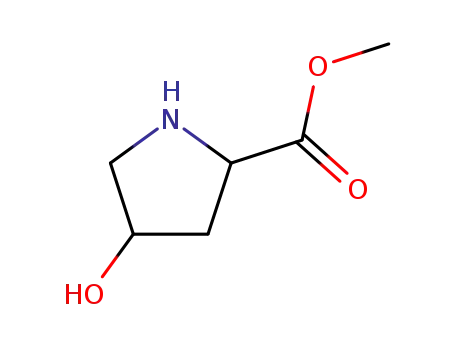
4(R)-hydroxyproline methyl ester
-
2443-30-3
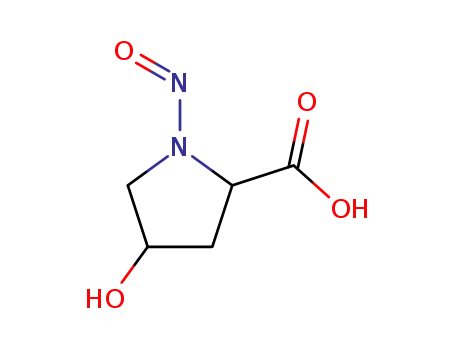
N-nitrosohydroxyproline
-
7732-18-5
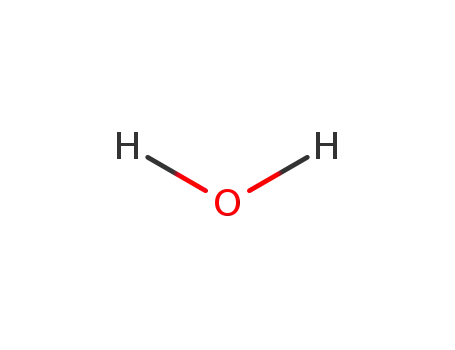
water
-
609-36-9
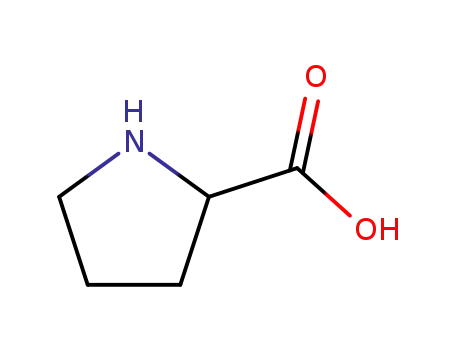
rac-Pro-OH
51-35-4 Downstream products
-
110-80-5
2-ethoxy-ethanol
-
2443-30-3

N-nitrosohydroxyproline
-
640-61-9
N-methyl-p-toluenesulfonylamide
-
1060936-14-2
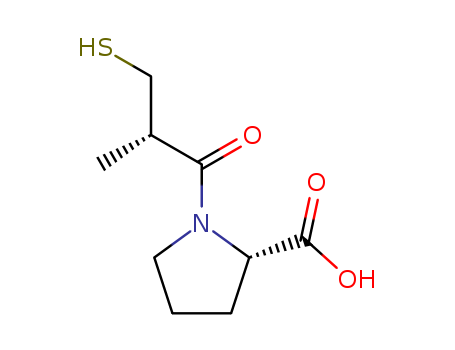
1-Amino-1-carboxy-2-methyl-propane-2-thiol anion
Relevant Products
-
Tesamorelin
CAS:218949-48-5
-
Captopril
CAS:62571-86-2
-
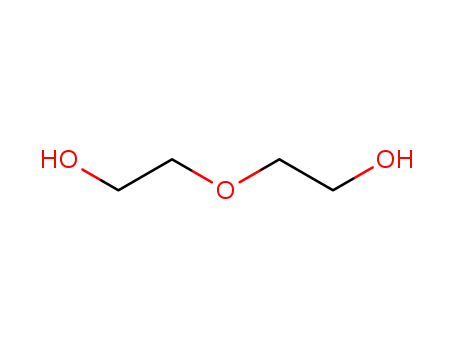
Diethylene glycol
CAS:111-46-6