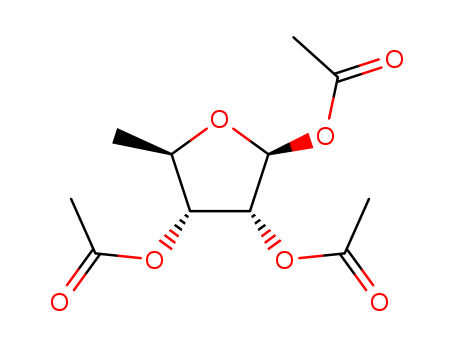
62211-93-2
- Product Name:1,2,3-Triacetyl-5-deoxy-D-ribose
- Molecular Formula:C11H16O7
- Purity:99%
- Molecular Weight:260.244
Product Details;
CasNo: 62211-93-2
Molecular Formula: C11H16O7
Manufacturer supply 1,2,3-Triacetyl-5-deoxy-D-ribose 62211-93-2 with sufficient stock and high standard
- Molecular Formula:C11H16O7
- Molecular Weight:260.244
- Vapor Pressure:0.00044mmHg at 25°C
- Melting Point:63-64°C
- Refractive Index:1.465
- Boiling Point:315.3 °C at 760 mmHg
- Flash Point:135.7 °C
- PSA:88.13000
- Density:1.23 g/cm3
- LogP:0.15770
1,2,3-Triacetyl-5-deoxy-D-ribose(Cas 62211-93-2) Usage
|
Chemical Composition and Structure |
1,2,3-Tri-O-acetyl-5-deoxy-beta-D-ribofuranose is an organic compound derived from ribofuranose, a five-carbon sugar. It is characterized by acetyl groups (CH?CO-) replacing the hydroxyl groups (OH) at positions 1, 2, and 3 of ribofuranose, and the absence of the hydroxyl group at position 5. It specifically refers to the β-configuration D-ribofuranose. |
|
Mechanism of Action |
As a fluoropyrimidine prodrug, 1,2,3-Tri-O-acetyl-5-deoxy-beta-D-ribofuranose is converted into active metabolites that inhibit DNA synthesis and exhibit antitumor properties. These metabolites interfere with the replication of cancer cells, leading to their destruction. |
InChI:InChI=1/C11H16O7/c1-5-9(16-6(2)12)10(17-7(3)13)11(15-5)18-8(4)14/h5,9-11H,1-4H3/t5-,9-,10-,11+/m1/s1
62211-93-2 Relevant articles
Preparation method of capecitabine intermediate
-
Paragraph 0007; 0016-0017, (2019/12/02)
The invention discloses a preparation me...
Preparation method of high-purity capecitabine key intermediate
-
Paragraph 0055; 0056; 0057, (2019/03/06)
The invention discloses a preparation me...
A three-acetyl deoxyribose α isomer preparation method
-
Paragraph 0015; 0016, (2019/07/04)
The invention discloses a capecitabine i...
AMPHIPHILE PRODRUGS
-
, (2019/06/12)
Amphiphilic prodrugs of general formula ...
62211-93-2 Process route
-
-
279673-09-5,947605-23-4
(2R,3R,4S,5R)-5-methyltetrahydrofuran-2,3,4-triol

-
-
108-24-7
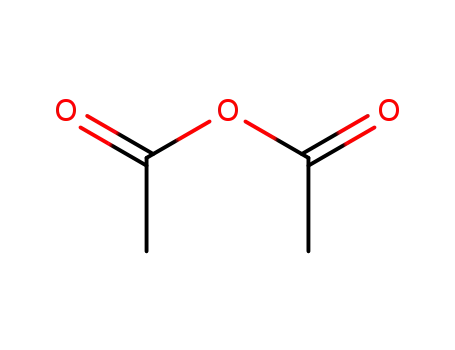
acetic anhydride

-
-
27821-07-4,27930-18-3,37076-71-4,62211-93-2,63903-44-6,76497-54-6
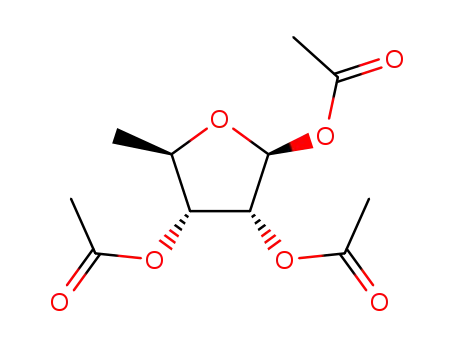
1,2,3-tri-O-acetyl-5-deoxy-β-D-ribofuranose
| Conditions | Yield |
|---|---|
|
With
dmap; triethylamine;
at -5 - 5 ℃;
for 4h;
|
86.4% |
|
With
dmap; triethylamine;
at 5 - 15 ℃;
|
78.2% |
|
With
pyridine; 1H-imidazole;
at 20 ℃;
for 6h;
|
|
|
With
dmap; triethylamine;
at -5 - 5 ℃;
for 4h;
Large scale;
|
-
-
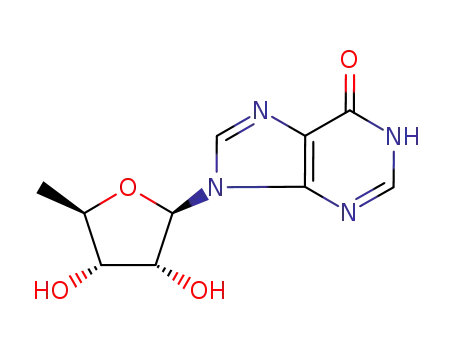
5'-deoxyinosine

-

-
108-24-7
acetic anhydride

-

-
27821-07-4,27930-18-3,37076-71-4,62211-93-2,63903-44-6,76497-54-6
1,2,3-tri-O-acetyl-5-deoxy-β-D-ribofuranose
| Conditions | Yield |
|---|---|
|
for 9h;
Reflux;
|
86.68% |
|
5'-deoxyinosine; acetic anhydride;
at 100 ℃;
for 1h;
With
boric acid;
for 10h;
|
84.1% |
|
With
boric acid;
at 100 ℃;
for 11h;
|
84.1% |
62211-93-2 Upstream products
-
158112-55-1
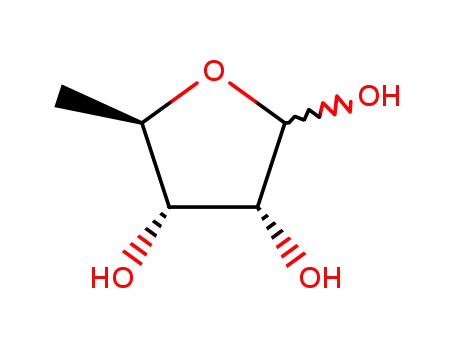
5-O-deoxy-D-ribofuranose
-
108-24-7

acetic anhydride
-
13039-75-3
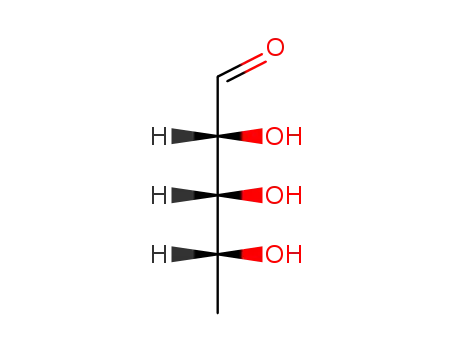
5′-deoxyribose
-
23202-81-5
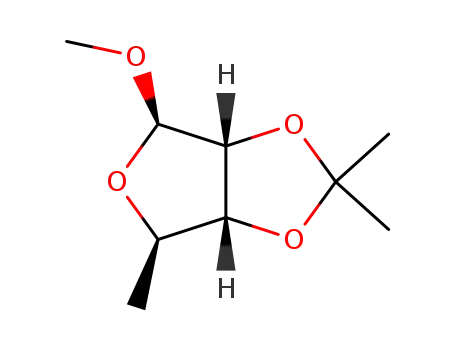
methyl 5-deoxy-2,3-O-isopropylidene-β-D-ribofuranoside
62211-93-2 Downstream products
-
161599-46-8
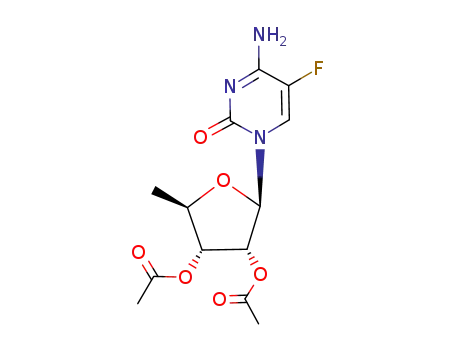
(2R,3R,4R,5R)-2-(4-amino-5-fluoro-2-oxopyrimidin-1(2H)-yl)-5-methyl-tetrahydrofuran-3,4-diyl diacetate
-
154361-50-9
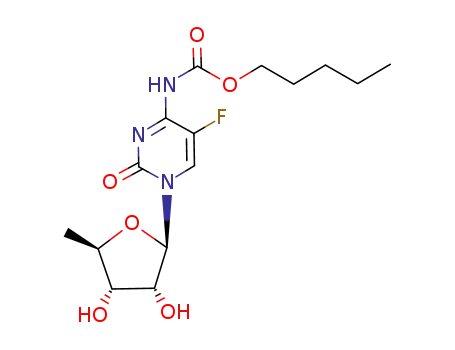
capecitabine
-
279673-09-5

(2R,3R,4S,5R)-5-methyltetrahydrofuran-2,3,4-triol
-
93978-94-0
5-deoxy-α-D-ribofuranose
Relevant Products
-
Tesamorelin
CAS:218949-48-5
-
DSIP
CAS:62568-57-4
-
Selank
CAS:129954-34-3