104-15-4
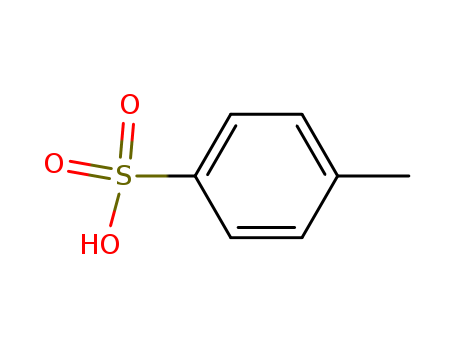
- Product Name:p-Toluenesulfonic acid
- Molecular Formula:C7H8O3S
- Purity:99%
- Molecular Weight:172.205
Product Details;
CasNo: 104-15-4
Molecular Formula: C7H8O3S
Appearance: clear colorless to light yellow solution
Reputable supplier selling p-Toluenesulfonic acid 104-15-4 with stock
- Molecular Formula:C7H8O3S
- Molecular Weight:172.205
- Appearance/Colour:clear colorless to light yellow solution
- Vapor Pressure:69.8Pa at 20℃
- Melting Point:106~107 °C
- Refractive Index:1.3825-1.3845
- Boiling Point:116 °C
- PKA:-0.43±0.50(Predicted)
- Flash Point:41 °C
- PSA:62.75000
- Density:1.34 g/cm3
- LogP:2.32250
p-Toluenesulfonic acid(Cas 104-15-4) Usage
|
Chemical Description |
p-toluenesulfonic acid is an organic compound with the formula CH3C6H4SO3H. |
|
Overview |
p-toluene sulfonic acid (Its molecular structural formula is p-CH3C6H4SO3H, also known as TsOH, English name is p-toluene sulfonic acid) referred to as PTS, is a non-oxidizing organic acid, white needle or powder crystals, soluble in water, alcohols, ethers and other polar solvents. Easy deliquescence, easy to make wood, cotton fabric dehydration and carbonization, insoluble in benzene and toluene. generating p-cresol when alkali fusion. Commonly, p-toluenesulfonic acid-monohydrate (TsOHH2O) or tetrahydrate (TsOH4H2O) is preferred. Preparation of methyl p-cresol acid in industry is by using concentrated sulfuric acid on the toluene sulfonation of p-toluenesulfonic acid. The preparated p-toluenesulfonic acid often contains benzene sulfonic acid and sulfuric acid impurities, can be purified in recrystallization of concentrated hydrochloric acid, azeotropic drying. p-toluene sulfonic acid is widely used as catalyst agent in the synthesis of pharmaceuticals, pesticides, polymerization stabilizer and organic synthesis (esters, etc.), paint intermediates and resin curing agent. And it is also the commonly used acid catalyst in organic synthesis. It is neutralized with sodium hydroxide and then obtains sodium p-toluene sulfonate, and react with phosphorus pentachloride, can obtains p-toluenesulfonyl chloride. The latter used in the nucleophilic substitution reaction, also used as alcohol hydroxyl protective group. p-CH3C6H4SO3Na + PCl5 →p-CH3C6H4SO2Cl. The use of p-toluenesulfonic acid also catalyzes the protection of dihydrofuran on the alcohol, carboxylic acid esterification, transesterification reaction, making the aldehyde generate acetal. |
|
Refer to quality standards |
Item/Index Industrial Grade Pharmaceutical Grade Refined Grade Reagent Grade (Chemically Pure) Content (as C7H8O3S ? H2O)% ≥ 90-93.0 96.0 97.0 98.0 Free acid (H2SO4) ≤% 3.0 0.7 0.5 0.1 Moisture (excluding crystal water) ≤% 4.0 3.5 2.5 1.5 Iron (in Fe ++) ≤ ppm 50 30 30 10 Ignition residue ≤%/0.2 0.2 0.02 Melting point (°C)// 102-105 Ethanol dissolved test/qualified qualified qualified Water dissolution test/qualified qualified qualified |
|
Chemical properties |
Colorless monoclinic sheet or columnar crystals. Soluble in ethanol and ether, slightly soluble in water and hot benzene. |
|
Production method |
By p-toluenesulfonyl chloride hydrolysis derived. Toluene can also be used as raw materials, sulfonated by sulfuric acid derived. |
|
Hazards & Safety Information |
Category:Corrosive articles Toxicity classification:Low toxicity Acute toxicity Oral-rat LD50: 2480 mg/kg Flammability hazard characteristics Combustible; fire in the release of toxic sulfur dioxide gas Storage and transportation characteristics Storehouse is ventilates, low temperature and dry; Store with base separately Extinguishing agent Mist water, carbon dioxide, foam |
|
Definition |
ChEBI: p-Toluenesulfonic acid is an arenesulfonic acid that is benzenesulfonic acid in which the hydrogen at position 4 is replaced by a methyl group. It is a member of toluenes and an arenesulfonic acid. It is a conjugate acid of a toluene-4-sulfonate. |
|
Reactions |
p-Toluene sulfonic acid may be converted to p-toluene sulfonic anhydride by heating with phosphorus pentoxide. When TsOH is heated with acid and water, a hydrolysis reaction takes place and toluene is formed: CH3C6H4SO3H + H2O → C6H5CH3 + H2SO4 This reaction is general for aryl sulfonic acids, but the rate at which it occurs depends upon the structure of the acid, the temperature and the nature of the catalyzing acid. For example p- TsOH is unaffected by cold concentrated hydrochloric acid, but hydrolyzes when heated to 186°C in concentrated phosphoric acid. |
|
Flammability and Explosibility |
Nonflammable |
|
Preparation and handling |
TsOH is prepared on an industrial scale by the sulfonation of toluene. It hydrates readily. Common impurities include benzene sulfonic acid and sulfuric acid. Impurities can be removed by recrystallization from its concentrated aqueous solution followed by azeotropic drying with toluene. Toluene sulfonic acid finds use in organic synthesis as an "organic - soluble" acid catalyst. Examples of uses : Acetalization of an aldehyde. Esterification of carboxylic acids. Trans esterification of an ester. |
|
Tosylate esters |
Tosylate esters are used as alkylating agents because the tosyl group is electron-with drawing, which makes the tosylate anion a good leaving group. The tosyl group is also a protecting group for alcohols and amines, prepared by combining the alcohol with 4- toluenesulfonyl chloride, usually in an aprotic solvent, often pyridine, the basicity of which activates the reaction. Toluenesulfonate esters undergo nucleophilic attack or elimination. Reduction of tosylate esters gives the hydrocarbon. Thus, tosylation followed by reduction allows for the deoxygenation of alcohols. |
InChI:InChI=1/C7H8O3S/c1-6-2-4-7(5-3-6)11(8,9)10/h2-5H,1H3,(H,8,9,10)
104-15-4 Relevant articles
The mechanisms of acid-catalyzed hydrolysis of n-(4-substituted arylthio) phthalimides
Kutuk, Halil,Yakan, Hasan
, p. 1460 - 1469 (2011)
The acid-catalyzed hydrolysis of N-(4-su...
Effect of Amine Nature on Reaction Rate and Mechanism in Nucleophilic Substitution Reactions of 2,4-Dinitrophenyl X-Substituted Benzenesulfonates with Alicyclic Secondary Amines
Um, Ik-Hwan,Chun, Sun-Mee,Chae, Ok-Mi,Fujio, Mizue,Tsuno, Yuho
, p. 3166 - 3172 (2004)
Second-order rate constants have been me...
Evidence for complexes of different stoichiometries between organic solvents and cyclodextrins
Garcia-Rio,Herves,Leis,Mejuto,Perez-Juste,Rodriguez-Dafonte
, p. 1038 - 1048 (2006)
The influence of the organic solvent on ...
Removal of electrophilic potential genotoxic impurities using nucleophilic reactive resins
Lee, Claire,Helmy, Roy,Strulson, Christopher,Plewa, Jolanta,Kolodziej, Elizabeth,Antonucci, Vincent,Mao, Bing,Welch, Christopher J.,Ge, Zhihong,Al-Sayah, Mohammad A.
, p. 1021 - 1026 (2010)
Potential genotoxic impurities (PGI) are...
ESR study of free radical decomposition of N,N-bis(arylsulfonyl)hydroxylamines in organic solution
Balakirev, Maxim Yu.,Khramtsov, Valery V.
, p. 7263 - 7269 (1996)
Decomposition of N,N-bis(p-tolylsulfonyl...
-
Hisada et al.
, p. 2814 (1972)
-
Investigation of micellar media containing ?2-cyclodextrins by means of reaction kinetics: Basic hydrolysis of N-methyl-N-nitroso-p-toluenesulfonamide
Garciì?a-Riì?o,Leis,Mejuto,Peì?rez-Juste
, p. 7383 - 7389 (1997)
The kinetics of the basic hydrolysis of ...
Hydrogenolysis of 2-tosyloxy-1,3-propanediol into 1,3-propanediol over Raney Ni catalyst
Zheng, Zhi,Wang, Jianli,Lu, Zhen,Luo, Min,Zhang, Miao,Xu, Lixin,Ji, Jianbing
, p. 385 - 391 (2013)
2-Tosyloxy-1,3-propanediol (TPD), a pote...
-
Lee et al.
, p. 206,212 (1959)
-
The quest for sulfoquinone imine intermediates in the reaction of sulfanilic acid derivatives with nucleophiles
Thea, Sergio,Vigo, Daniele,Cevasco, Giorgio
, p. 611 - 614 (2002)
Data from kinetic and trapping studies s...
Influence of colloid suspensions of humic acids on the alkaline hydrolysis of N-methyl-N-nitroso-p-toluene sulfonamide
Astray,Garcia-Rio,Lodeiro,Mejuto,Moldes,Morales,Moyano
, p. 316 - 322 (2010)
The influence of humic substances (HSs) ...
General, fast, and high yield oxidation of thiols and disulfides to sulfonic and sulfinic acids using HOF·CH3CN
Shefer, Neta,Carmeli, Mira,Rozen, Shlomo
, p. 8178 - 8181 (2007)
Thiols and disulfides are oxidized to th...
Hypervalent iodine in synthesis. XXI: A facile method for the preparation of thiosulfonic S-esters by the oxidation of diaryl disulfides or thiophenols with phenyliodine(III) bis(trifluoroacetate)
Xia, Min,Chen, Zhen-Chu
, p. 1301 - 1308 (1997)
Phenyliodine(III) bis(trifluoroacetate) ...
-
Fritz,Gillette
, p. 1777,1778 (1968)
-
Competitive electron transfers from a tyrosyl side-chain and peptide bond in the photodegradation of N-tosyl α-aminomethylamides: An insight into photosynthesis and photodamage in the biological oxidation of water?
Hill, Roger R.,Moore, Sharon A.,Roberts, David R.
, p. 2838 - 2839 (2003)
Photo-excited N-tosyl derivatives of phe...
A general and efficient method for the preparation of organic sulfonic acids by insertion of sulfur trioxide into the metal-carbon bond of organolithiums
Smith, Keith,Hou, Duanjie
, p. 1530 - 1532 (1996)
-
-
Thomas,Anzilotti,Hennion
, p. 408 (1940)
-
Formation of o-nitrosobenzaldehyde from hydrolysis of o-nitrobenzyl tosylate. Evidence of intramolecular nucleophilic interaction
Chen, Ling-Jen,Burka, Leo T.
, p. 5351 - 5354 (1998)
Hydrolysis of o-nitrobenzyl tosylate in ...
Palladium nanoparticles as reusable catalyst for the synthesis of N-aryl sulfonamides under mild reaction conditions
Khalaj, Mehdi,Ghazanfarpour-Darjani, Majid,Talei Bavil Olyai, Mohamad Reza,Shamami, Sakineh Faraji
, p. 211 - 221 (2016)
An efficient palladium nanoparticles-cat...
Experimental and molecular modelling studies on aromatic sulfonation
Morley, John O.,Roberts, David W.,Watson, Simon P.
, p. 538 - 544 (2002)
The mechanism of the sulfonation of tolu...
Photodegradation of aryl sulfonamides: N-tosylglycine
Hill, Roger R.,Jeffs, Graham E.,Roberts, David R.,Wood, Sharon A.
, p. 1735 - 1736 (1999)
Continuing uncertainty about pathways an...
-
Schenk et al.
, p. 907,911 (1950)
-
A novel method for sulfonation of aromatic rings with silica sulfuric acid
Hajipour, Abdol R.,Mirjalili, Bi Bi F.,Zarei, Amin,Khazdooz, Leila,Ruoho
, p. 6607 - 6609 (2004)
Direct and chemoselective sulfonation of...
Regioselective Sulfonation of Aromatic Compounds over 1,3-Disulfonic Acid Imidazolium Chloride under Aqueous Media
Moosavi-Zare, Ahmad Reza,Zolfigol, Mohammad Ali,Noroozizadeh, Ehsan
, p. 1682 - 1684 (2016)
1,3-Disulfonic acid imidazolium chloride...
Reactions of the Nickel(I) Octaethylisobacteriochlorin Anion with Alkyl Halides
Stolzenberg, Alan M.,Stershic, Matthew T.
, p. 5397 - 5403 (1988)
Reactions of NiI(OEiBC)- with alkyl hali...
A Novel Concept of Acid Proliferation. Autocatalytic Fragmentation of an Acetoacetate Derivative as an Acid Amplifier
Ichimura, Kunihiro,Arimitsu, Koji,Kudo, Kazuaki
, p. 551 - 552 (1995)
tert-Butyl 2-methyl-2-(p-toluenesulfonyl...
-
Hisada et al.
, p. 2035 (1972)
-
Cholesteryl Tosylate: A Solvolytic Investigation
Roberts, Donald D.
, p. 1269 - 1272 (1993)
-
Suzuki Cross-Coupling for the incorporation of labeled methyl groups onto aryl halides. A synthesis of [14C]Tosyl chloride and its use in the synthesis of [14C]L-738,167
Braun, Matthew P.,Dean, Dennis C.,Melillo, David G.
, p. 469 - 476 (1999)
A synthesis of [4-methyl-14C]tosyl chlor...
Bis Sulfate as an Organosilicon Synthon
Voronkov, M. G.,Roman, V. K.,Maletina, E. A.
, p. 277 - 280 (1982)
-
Potential photoacid generators based on oxime sulfonates
Plater, M. John,Harrison, William T. A.,Killah, Ross
, p. 26 - 33 (2019)
The bis-oxime of acenaphthenequinone and...
Neighboring group competition revisited: Relative abilities of cyclobutyl/cyclopentyl/phenyl groups to stabilize an electron-deficient carbon
Roberts
, p. 1341 - 1343 (1999)
-
-
Englund,Aries,Othmer
, p. 189,193 (1953)
-
Importance of repulsion of lone electron pairs in the enhanced reactivity of 1,8-naphthyridine and the large α-effect of hydrazine in the aminolyses of p-toluenesulfonyl chloride
Oae, Shigeru,Kadoma, Yoshihito
, p. 1184 - 1188 (1986)
The rates of aminolyses of p-toluenesulf...
Hydrolysis of some imidazole, benzimidazole, and 1,2,3-benzotriazole derivatives according to HPLC and NMR diffusimetry data
Polyakova,Bulanova,Vartapetyan
, p. 820 - 822 (2001)
Hydrolysis of 1-mesylimidazole, 1-mesylb...
A kinetic study of acid-catalyzed hydrolysis of some arylsulfonyl phthalimides
Kutuk, Halil,Ozturk, Seyhan
, p. 332 - 340 (2009)
The acid-catalyzed hydrolysis of arylsul...
Hydrolysis Mechanism of Alkynyl Benzoates, Tosylates, and Phosphates
Allen, Annette D.,Kitamura, Tsugio,Roberts, Kenneth A.,Stang, Peter J.,Tidwell, Thomas T.
, p. 622 - 624 (1988)
-
Extremely mild and selective method for hydrolysis of tosyl esters by photo-sensitized single electron transfer reactions
Nishida,Hamada,Yonemitsu
, p. 2977 - 2980 (1990)
-
Recyclable imidazolium ion-tagged nickel catalyst for microwave-assisted C-S cross-coupling in water using sulfonyl hydrazide as the sulfur source
Saini, Vaishali,Khungar, Bharti
, p. 12796 - 12801 (2018)
Herein, we report the facile and conveni...
Photocleavage of o-nitrobenzyl ether derivatives for rapid biomedical release applications
Kim, Moon Suk,Diamond, Scott L.
, p. 4007 - 4010 (2006)
The externally controlled cleavage of co...
Solvolyses of Secondary Sulfonates in Aqueous Ethanol and Acetone. Nonlinear mY Relationships due to Leaving Group and Medium Effects
Bentley, William T.,Bowen, Christine T.,Brown, Herbert C.,Chloupek, Frank J.
, p. 38 - 42 (1981)
Solvolitic rate constants for secondary ...
BRANCHED AMINO ACID SURFACTANTS FOR AGRICULTURAL PRODUCTS
-
, (2022/01/23)
-
BRANCHED AMINO ACID SURFACTANTS
-
, (2022/01/24)
The present disclosure provides derivati...
BRANCHED AMINO ACID SURFACTANTS FOR PERSONAL CARE AND COSMETIC PRODUCTS
-
, (2022/01/24)
-
Primary Sulfonamide Functionalization via Sulfonyl Pyrroles: Seeing the N?Ts Bond in a Different Light
Ozaki, Tomoya,Yorimitsu, Hideki,Perry, Gregory J. P.
supporting information, p. 15387 - 15391 (2021/10/04)
Despite common occurrence in molecules o...
104-15-4 Process route
-
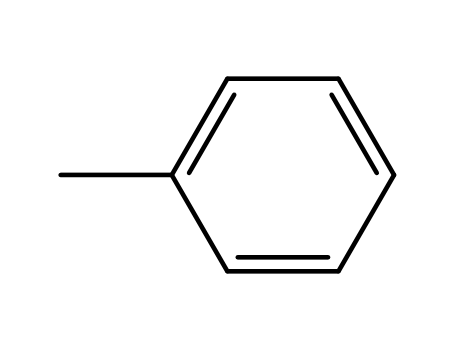
-
108-88-3,15644-74-3,16713-13-6
toluene

-
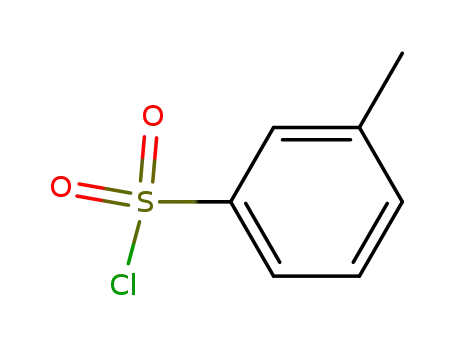
-
1899-93-0
3-methylphenylsulfonyl chloride

-
-
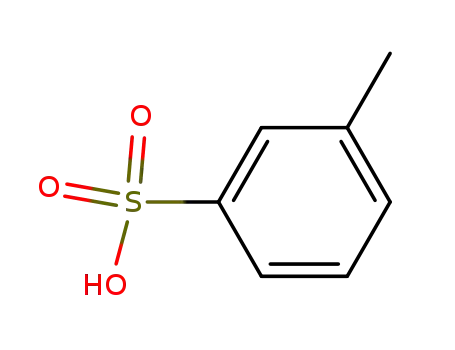
617-97-0
m-toluenesulfonic acid

-
-
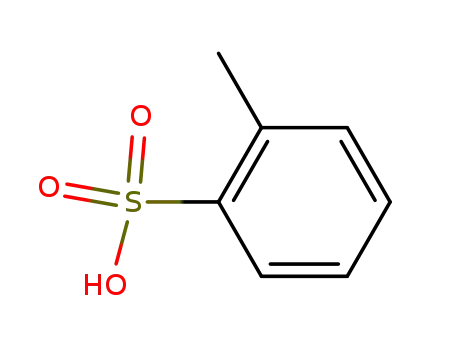
88-20-0
o-toluenesulfonic acid

-
-
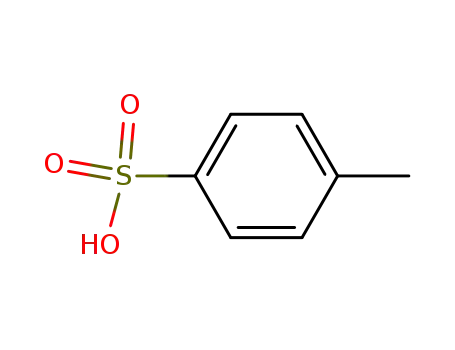
104-15-4
toluene-4-sulfonic acid

-
-
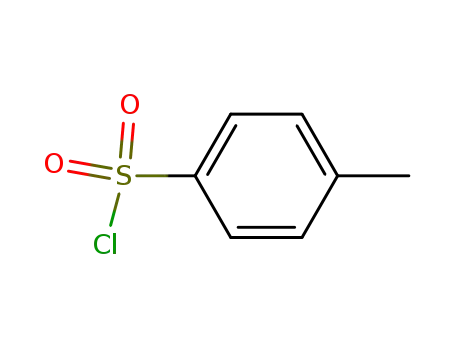
98-59-9
p-toluenesulfonyl chloride

-
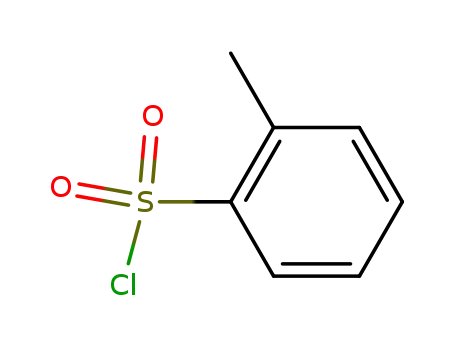
-
133-59-5
Toluene-2-sulfonyl chloride
| Conditions | Yield |
|---|---|
|
With
chlorosulfonic acid;
at 25 ℃;
for 1.5h;
Product distribution;
|
11.6 % Chromat. 3.2 % Chromat. 85.2 % Chromat. 16.1 % Chromat. 1.8 % Chromat. 82.1 % Chromat. |
-
-
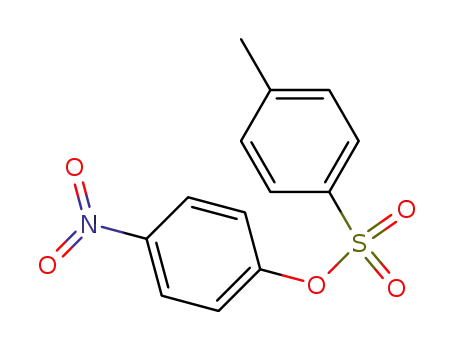
1153-45-3
4-nitrophenyl 4-methylbenzenesulfonate

-
-
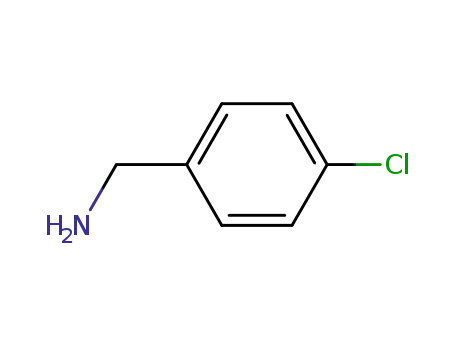
104-86-9
4-chlorobenzylamine

-
-
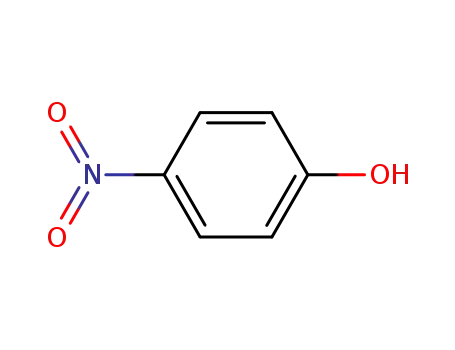
100-02-7,78813-13-5,89830-32-0
4-nitro-phenol

-
-
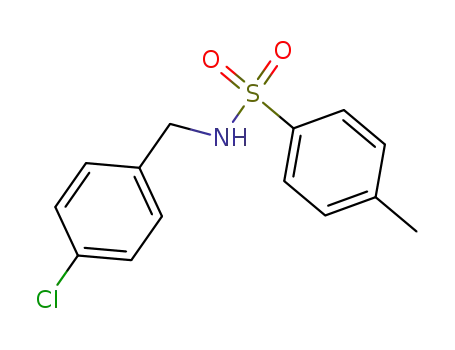
10504-98-0
N-(4-chlorobenzyl)-p-toluenesulfonamide

-

-
104-15-4
toluene-4-sulfonic acid

-
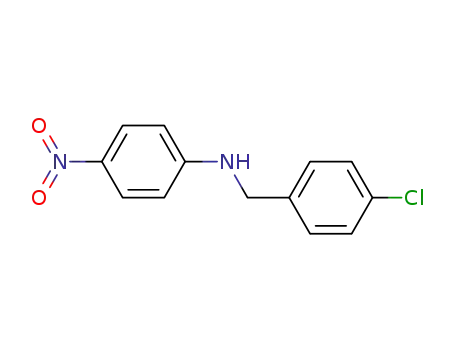
-
N-(4-chlorobenzyl)-4-nitroaniline
| Conditions | Yield |
|---|---|
|
In
acetonitrile;
at 65 ℃;
Kinetics;
|
104-15-4 Upstream products
-
536-57-2
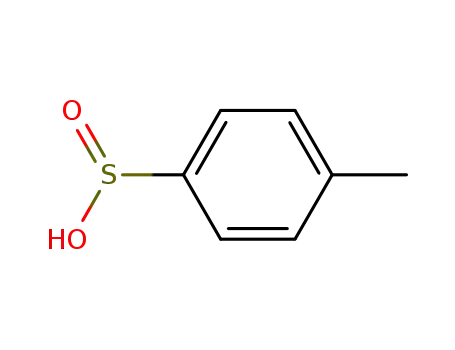
p-toluene sulfinic acid
-
57573-52-1

4-methylbenzene diazonium
-
60-29-7
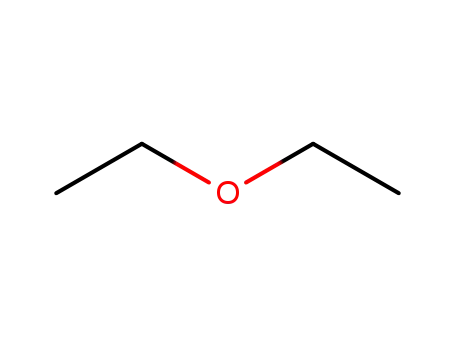
diethyl ether
-
2943-42-2
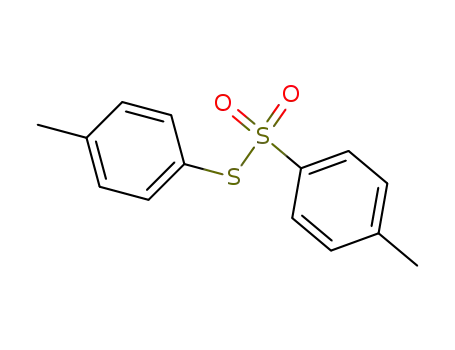
di(4-methyl)phenylthiosulfonate
104-15-4 Downstream products
-
101169-52-2
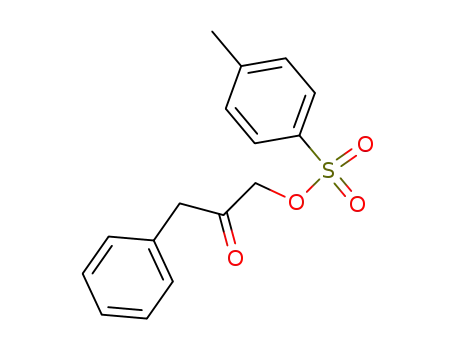
toluene-4-sulfonic acid 2-oxo-3-phenyl-propyl ester
-
15051-88-4
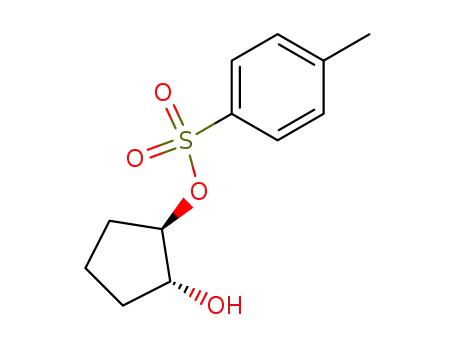
trans-cyclopentane-1,2-diol 1-tosylate
-
7716-07-6
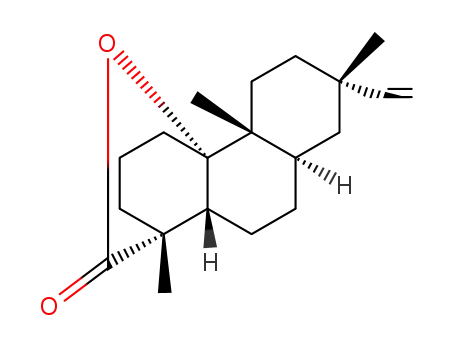
(13S)-10-hydroxy-5β,10α-ros-15-en-18-oic acid-lactone
-
39794-75-7
ethyl α-p-toluenesulfonyloxyacetate
Relevant Products
-
Tesamorelin
CAS:218949-48-5
-
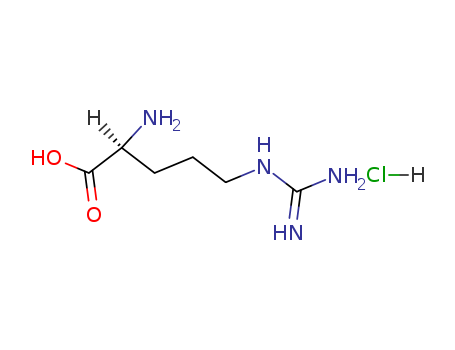
2-Amino-5-guanidinovaleric acid monohydrochloride
CAS:1119-34-2
-
Carboplatin
CAS:41575-94-4