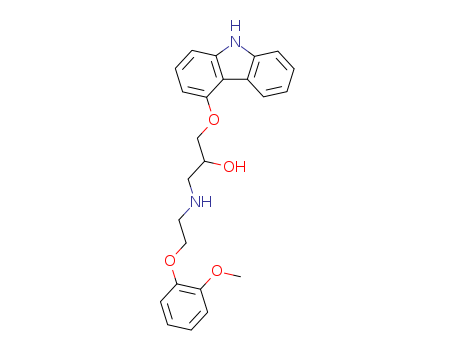
72956-09-3
- Product Name:Carvedilol
- Molecular Formula:C24H26N2O4
- Purity:99%
- Molecular Weight:496.517
Product Details;
CasNo: 72956-09-3
Molecular Formula: C24H26N2O4
Appearance: colourless crystalline powder
Manufacturer Sells Best Quality Carvedilol 72956-09-3 with stock
- Molecular Formula:C24H26N2O4
- Molecular Weight:496.517
- Appearance/Colour:colourless crystalline powder
- Vapor Pressure:1.14E-32mmHg at 25°C
- Melting Point:113-117 °C
- Boiling Point:655.2 °C at 760 mmHg
- PKA:13.90±0.20(Predicted)
- Flash Point:350.1 °C
- PSA:75.74000
- Density:1.25 g/cm3
- LogP:4.12890
Carvedilol(Cas 72956-09-3) Usage
|
Manufacturing Process |
1-(9H-Carbazol-4-yloxy)-3-((2-(2-methoxyphenoxy)ethyl)amino)-2-propanol may be synthesized by the method of preparation of S-(-)-(1-carbazol-4- yloxy)-3-[2-(2-methoxyphenoxy)]ethylaminopropan-2-ol (Patent US 4,697,022 and 4,824,963).27.5 g 4-hydroxycarbazole are dissolved in a mixture of 150 ml 1 N aqueous sodium hydroxide solution and 70 ml dimethylsulfoxide. To this is added at ambient temperature 13.9 g epichlorohydrin, followed by stirring for 18 hours at ambient temperature. 280 ml water are then added thereto, followed by stirring for 15 min and filtering off with suction. The filter residue is washed with 0.1 N aqueous sodium hydroxide solution and water and subsequently dissolved in methylene chloride. The methylene chloride solution is dried over anhydrous sodium sulfate, treated with active charcoal and floridin and evaporated. 4-(2,3-Epoxypropoxy)-carbazole is purified by recrystallising twice from ethyl acetate. From the mother liquors there are isolated a further 4- (2,3-epoxypropoxy)-carbazole.10 g 4-(2,3-epoxypropoxy)-carbazole are, together with 13.97 g o-methoxyphenoxyethylamine, heated under reflux in 70 ml isopropanol for 2 hours. The solvent is evaporated off and the residue is stirred for 2 hours with a mixture of 115 ml toluene, 35 ml cyclohexane and 40 ml ethyl acetate. After filtering off with suction, the (1-carbazol-4-yloxy)-3-[2-(2-methoxyphenoxy)]-ethylaminopropan-2-ol is recrystallised from 150 ml ethyl acetate. |
|
Therapeutic Function |
Beta-adrenergic blocker |
|
Biological Activity |
Potent β -adrenoceptor and α 1 -adrenoceptor antagonist (K i values are 0.81, 0.96 and 2.2 nM for β 1 -, β 2 - and α 1 -adrenoceptors respectively) that displays antihypertensive and peripheral vasodilatory activity. Blocks cardiac inward-rectifier K + (K IR ) channels, voltage-dependent Ca 2+ channels and exhibits antioxidant properties at higher concentrations. |
|
Biochem/physiol Actions |
Cavedilol is a non-selective β-adrenergic blocker with α1 blocking activity. Carvedilol is used specifically for the treatment of heart failure and high blood pressure. It has been shown to improve left ventricular ejection fraction and may reduce mortality. |
|
Veterinary Drugs and Treatments |
Carvedilol may be useful as adjunctive therapy in the treatment of heart failure (dilated cardiomyopathy) in dogs. There is a fair amount of controversy at present among veterinary cardiologists as to whether this drug will find a therapeutic niche. |
|
in vitro |
carvedilol potently inhibited fe2+-initiated lipid peroxidation in rat brain homogenate with an ic50 of 8.1 μm. in rat brain homogenate, carvedilol protected against fe2+-induced α-tocopherol depletion with an ic50 of 17.6 μm. carvedilol dose-dependently decreased the intensity of the dmpo-oh signal, with an ic50 of 25 μm [1]. carvedilol prevented vascular smooth muscle cell migration, proliferation, and neointimal formation following vascular injury. in human cultured pulmonary artery vascular smooth muscle cells, carvedilol (0.1-10 μm) concentration-dependently inhibited the mitogenesis stimulated by platelet-derived growth factor, epidermal growth factor, thrombin, and serum, with ic50 values ranging from 0.3 to 2.0 μm. carvedilol concentration-dependently inhibited vascular smooth muscle cell migration induced by platelet-derived growth factor with an ic50 value of 3 μm [3]. |
|
Drug interactions |
Potentially hazardous interactions with other drugs Anaesthetics: enhanced hypotensive effect. Analgesics: NSAIDs antagonise hypotensive effect. Anti-arrhythmics: increased risk of myocardial depression and bradycardia; increased risk of bradycardia, myocardial depression and AV block with amiodarone; increased risk of myocardial depression and bradycardia with flecainide. Antibacterials: concentration reduced by rifampicin. Antidepressants: enhanced hypotensive effect with MAOIs. Antihypertensives; enhanced hypotensive effect; increased risk of withdrawal hypertension with clonidine; increased risk of first dose hypotensive effect with post-synaptic alpha-blockers such as prazosin. Antimalarials: increased risk of bradycardia with mefloquine. Antipsychotics enhanced hypotensive effect with phenothiazines. Calcium-channel blockers: increased risk of bradycardia and AV block with diltiazem; hypotension and heart failure possible with nifedipine and nisoldipine; asystole, severe hypotension and heart failure with verapamil. Ciclosporin: increased trough concentration, reduce dose by 20% in affected patients. Cytotoxics: possible increased risk of bradycardia with crizotinib. Diuretics: enhanced hypotensive effect. Fingolimod: possibly increased risk of bradycardia. Moxisylyte: possible severe postural hypotension. Sympathomimetics: severe hypertension with adrenaline and noradrenaline and possibly with dobutamine. |
|
Metabolism |
Carvedilol is subject to considerable first-pass metabolism in the liver; the absolute bioavailability is about 25%. It is extensively metabolised in the liver, primarily by the cytochrome P450 isoenzymes CYP2D6 and CYP2C9, and the metabolites are excreted mainly in the bile. |
|
Definition |
ChEBI: A member of the class of carbazoles that is an adrenergic antagonist with non-selective beta- and alpha-1 receptor blocking properties which helps in the management of congestive heart failure. |
|
Brand name |
Coreg (GlaxoSmithKline);Dilatrend. |
|
General Description |
Carvedilol (Coreg) is a β-blocker that hasa unique pharmacological profile. Like labetalol, it is aβ-blocker that possesses α1-blocking activity. Only the(S) enantiomer possesses the β-blocking activity, althoughboth enantiomers are blockers of the α1-receptor. Overall,its β-blocking activity is 10- to 100-fold of its α-blocking activity. |
InChI:InChI=1/C24H26N2O4.C4H6O6/c1-28-21-10-4-5-11-22(21)29-14-13-25-15-17(27)16-30-23-12-6-9-20-24(23)18-7-2-3-8-19(18)26-20;5-1(3(7)8)2(6)4(9)10/h2-12,17,25-27H,13-16H2,1H3;1-2,5-6H,(H,7,8)(H,9,10)
72956-09-3 Relevant articles
In-situ and one-step preparation of protein film in capillary column for open tubular capillary electrochromatography enantioseparation
Li, Ling,Xue, Xuqi,Zhang, Huige,Lv, Wenjuan,Qi, Shengda,Du, Hongying,Manyande, Anne,Chen, Hongli
supporting information, p. 2139 - 2142 (2021/04/07)
In this work, the phase-transitioned BSA...
Drug repurposing and rediscovery: Design, synthesis and preliminary biological evaluation of 1-arylamino-3-aryloxypropan-2-ols as anti-melanoma agents
Chang, Qi,Long, Jing,Hu, Liqing,Chen, Zhuo,Li, Qianbin,Hu, Gaoyun
, (2020/04/09)
Malignant melanoma (MM) presents as the ...
Preparation and evaluation of a triazole-bridged bis(β-cyclodextrin)–bonded chiral stationary phase for HPLC
Shuang, Yazhou,Liao, Yuqin,Wang, Hui,Wang, Yuanxing,Li, Laisheng
, p. 168 - 184 (2019/11/25)
A triazole-bridged bis(β-cyclodextrin) w...
Hybridization of β-Adrenergic Agonists and Antagonists Confers G Protein Bias
Stanek, Markus,Picard, Louis-Philippe,Schmidt, Maximilian F.,Kaindl, Jonas M.,Hübner, Harald,Bouvier, Michel,Weikert, Dorothée,Gmeiner, Peter
, p. 5111 - 5131 (2019/05/28)
Starting from the β-adrenoceptor agonist...
72956-09-3 Process route
-
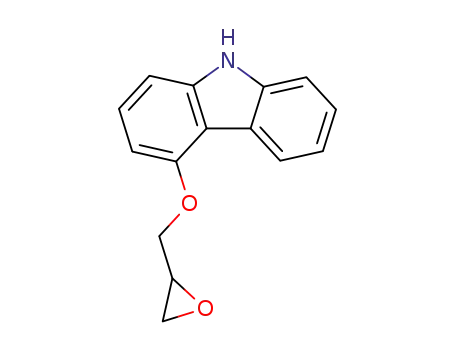
-
51997-51-4,95093-96-2
4-(2,3-epoxypropoxy)carbazole

-
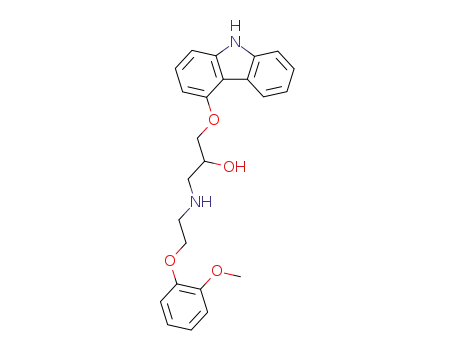
-
72956-09-3
carvedilol
| Conditions | Yield |
|---|---|
|
2-[(2-methoxy)phenoxy]ethylamine hydrochloride;
With
potassium carbonate;
In
isopropyl alcohol;
at 35 ℃;
for 0.25h;
4-(2,3-epoxypropoxy)carbazole;
In
isopropyl alcohol;
at 83 ℃;
for 5h;
|
45% |
|
Multi-step reaction with 3 steps
1: potassium carbonate / N,N-dimethyl-formamide / 4 h / 140 °C
2: methylamine / water / 2 h / 80 °C
3: potassium carbonate / toluene / 80 - 85 °C
With
potassium carbonate; methylamine;
In
water; N,N-dimethyl-formamide; toluene;
|
|
|
Multi-step reaction with 4 steps
1: hydrogenchloride / water; methanol / 0 - 30 °C
2: potassium carbonate / N,N-dimethyl-formamide / 2 h / 90 - 95 °C
3: methylamine / water / 2 h / 80 °C
4: dipotassium hydrogenphosphate; N-benzyl-N,N,N-triethylammonium chloride / toluene / 5 h / Reflux
With
hydrogenchloride; dipotassium hydrogenphosphate; N-benzyl-N,N,N-triethylammonium chloride; potassium carbonate; methylamine;
In
methanol; water; N,N-dimethyl-formamide; toluene;
|
|
|
Multi-step reaction with 3 steps
1: 4-methoxypyridine N-oxide; sodium sulfate / dichloromethane / 60 h / 20 °C
2: potassium carbonate / toluene / 60 h / Reflux
3: tetrabutyl ammonium fluoride / tetrahydrofuran / 17 h / 30 - 40 °C
With
4-methoxypyridine N-oxide; tetrabutyl ammonium fluoride; potassium carbonate; sodium sulfate;
In
tetrahydrofuran; dichloromethane; toluene;
|
|
|
Multi-step reaction with 2 steps
1: water; hydrogen bromide / isopropyl alcohol / 25 - 60 °C
2: triethylamine / isopropyl alcohol / 60 °C
With
water; hydrogen bromide; triethylamine;
In
isopropyl alcohol;
|
-
![(+/-)-1-(9H-carbazol-4-yloxy)-3-[[2-(2-methoxyphenoxy)-ethyl]-amino]-2-propanol salicylate](/upload/2025/4/2e23c478-cc81-49a6-8ca1-95f2e719678d.png)
-
787598-91-8
(+/-)-1-(9H-carbazol-4-yloxy)-3-[[2-(2-methoxyphenoxy)-ethyl]-amino]-2-propanol salicylate

-
-
918903-20-5
C39H39N3O6

-

-
72956-09-3
carvedilol
| Conditions | Yield |
|---|---|
|
With
sodium hydroxide;
In
water; isopropyl alcohol;
at 50 - 70 ℃;
for 0.75h;
|
91.21% |
|
(+/-)-1-(9H-carbazol-4-yloxy)-3-[[2-(2-methoxyphenoxy)-ethyl]-amino]-2-propanol salicylate;
With
triethylamine;
In
ethyl acetate;
at 10 - 35 ℃;
for 1h;
With
sodium chloride;
In
water; ethyl acetate;
for 0.5h;
|
72956-09-3 Upstream products
-
787598-91-8
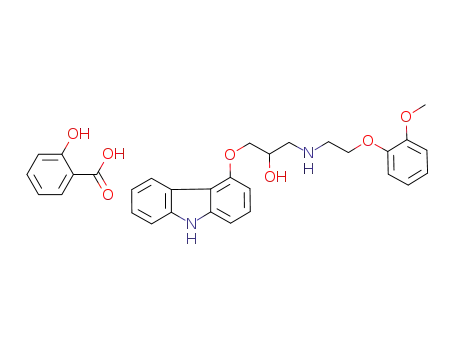
(+/-)-1-(9H-carbazol-4-yloxy)-3-[[2-(2-methoxyphenoxy)-ethyl]-amino]-2-propanol salicylate
-
64464-07-9
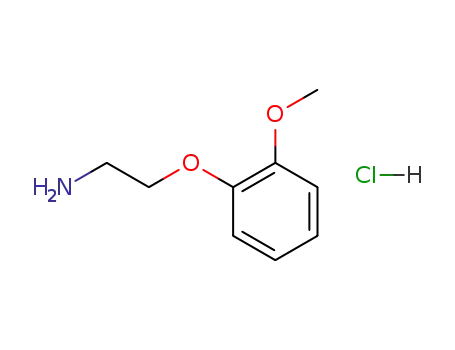
2-[(2-methoxy)phenoxy]ethylamine hydrochloride
-
51997-51-4

4-(2,3-epoxypropoxy)carbazole
-
1836-62-0
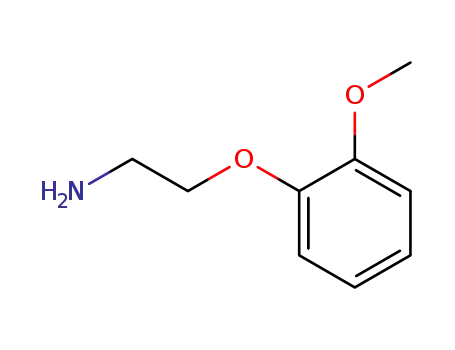
2-(2-methoxy-phenoxy)-ethylamine
72956-09-3 Downstream products
-
374779-45-0
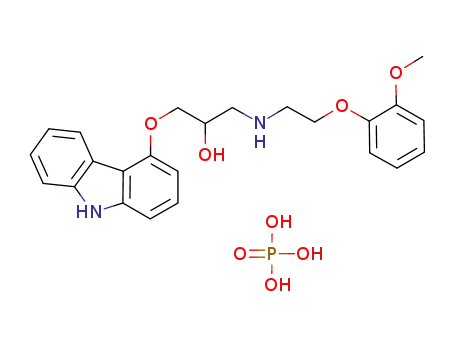
1-(9H-carbazole-4-oxy)-3-[2-(2-methoxyphenoxy)ethylamino]-2-propanolphosphate
-
852995-81-4
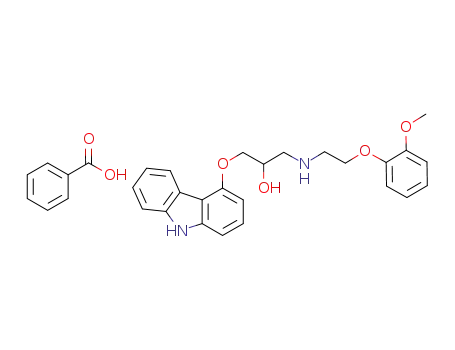
(+/-) 1-(9H-carbazol-4-yloxy)-3-[2-(2-methoxyphenoxy)ethyl]amino-2-propanol benzoate
-
610309-89-2
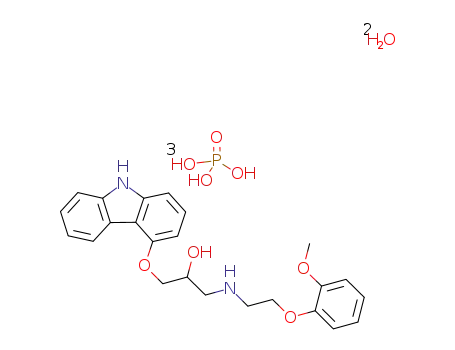
1-(carbazol-4-yloxy)-3-[[2-(O-methoxyphenoxy)ethyl]amino]-2-propanol dihydrogen phosphate dihydrate
-
610309-89-2
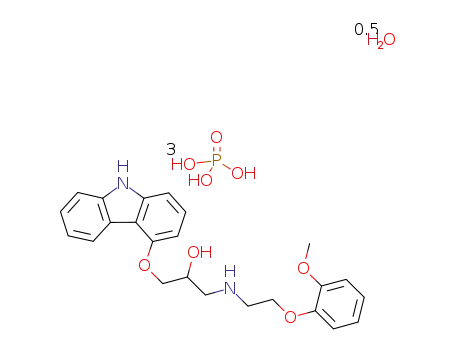
1-(carbazol-4-yloxy)-3-[[2-(O-methoxyphenoxy)ethyl]amino]-2-propanol dihydrogen phosphate hemihydrate
Relevant Products
-
Tesamorelin
CAS:218949-48-5
-
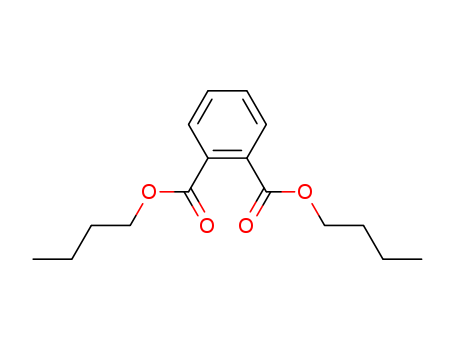
Dibutyl phthalate
CAS:84-74-2
-
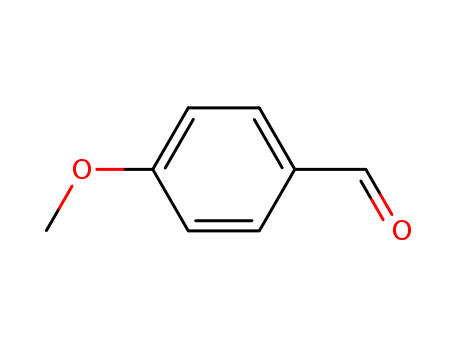
p-Anisaldehyde
CAS:123-11-5