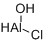
112-80-1
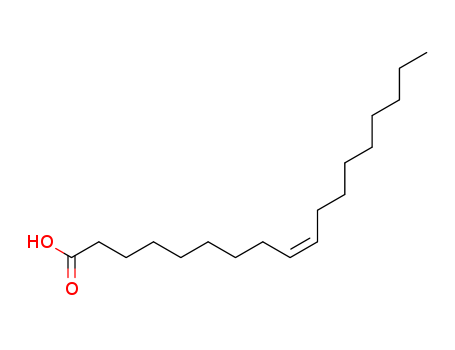
- Product Name:Oleic acid
- Molecular Formula:C18H34O2
- Purity:99%
- Molecular Weight:282.467
Product Details;
CasNo: 112-80-1
Molecular Formula: C18H34O2
Appearance: colourless to light yellow viscous liquid
Oleic acid Good Supplier In Bulk Supply High Purity 112-80-1
- Molecular Formula:C18H34O2
- Molecular Weight:282.467
- Appearance/Colour:colourless to light yellow viscous liquid
- Vapor Pressure:52 mm Hg ( 37 °C)
- Melting Point:13-14 °C(lit.)
- Refractive Index:n20/D 1.377
- Boiling Point:360 °C at 760 mmHg
- PKA:pKa 5.35(H2O,t =25) (Uncertain)
- Flash Point:270.1 °C
- PSA:37.30000
- Density:0.899 g/cm3
- LogP:6.10850
Oleic acid(Cas 112-80-1) Usage
|
Unsaturated fatty acid |
Oleic acid is a kind of unsaturated fatty acid with its molecular structure containing a carbon-carbon double bond, being the fatty acid that makes olein. It is one of the most extensive natural unsaturated fatty acids. Oil lipid hydrolysis can lead to oleic acid with the chemical formula being CH3 (CH2) 7CH = CH (CH2) 7 ? COOH. The glyceride of the oleic acid is one of the main ingredients of olive oil, palm oil, lard and other animal and vegetable oils. Its industrial products often contain 7~12% saturated fatty acids (palmitic acid, stearic acid) and a small amount of other unsaturated fatty acids (linoleic acid). It is colorless oily liquid with the specific gravity being 0.895 (25/25 ℃), freezing point of 4 ℃, the boiling point of 286 °C (13,332 Pa), and the refractive index of 1.463 (18 ° C). Its iodine value is 89.9 and its acidic value is 198.6. It is insoluble in water, but soluble in alcohol, benzene, chloroform, ether and other volatile oil or fixed oil. Upon exposure to air, especially when containing some impurities, it is susceptible to oxidation with its color turning into yellow or brown, accompanied with rancid odor. At normal pressure, it will be subject to decomposition 80~100 °C. It is manufactured through the saponification and acidification of animal and vegetable oils. Oleic acid is an indispensable nutrient in animal food. Its lead salt, manganese salt, cobalt salt belong to paint driers; its copper salt can be used as fish net preservatives; its aluminum salt can be used as the water repellent agent of fabric as well as the thickener of some lubricants. When being epoxidized, oleic acid can produce epoxy oleate (plasticizer). Upon subjecting to oxidative cracking, it can generate azelaic acid (raw material of polyamide resin). It can be sealed. Store it on darkness. Oleic acid exists in the animal and vegetable oil fat in large amount, being mainly in the form of glyceride. Some simple oleic esters can be applied to the textile, leather, cosmetics and pharmaceutical industries. The alkali metal salt of oleic acid can be dissolved in water, being one of the main components of soap. The lead, copper, calcium, mercury, zinc and other salts of oleic acid are soluble in water. It can be used as dry lubricants, paint drying agent and waterproofing agent. Oleic acid mainly comes from nature. Oil fat containing high content of oleic acid, after subjecting to saponification and acidification separation, can produce oleic acid. Oleic acid has cis-isomers. Natural oleic acids are all cis-structure (trans-structure oleic acid can’t be absorbed by the human body) with certain effect of softening the blood vessels. It also plays an important role in the metabolism process of human and animal. However, the oleic acid synthesized by the human body itself can’t meet the needs, so we need food intake. Thus, consumption of edible oil of high oleic acid content is healthy. The above information is edited Xiao Nan of lookchem. |
|
Physical and chemical properties |
Oleic acid, also known as cis-9-octadecenoic acid, being of chemical properties of single unsaturated carboxylic acid and is widely presented in animal and vegetable oils. For example, olive oil contains about 82.6%; peanut oil contains 60.0%; sesame oil contains 47.4%; soybean oil contains 35.5%; sunflower seed oil contains 34.0%; cottonseed oil contains 33.0%; rapeseed oil contains 23.9%; safflower oil contains 18.7%; the content in the tea oil can be as high as 83%; in animal oil: lard oil contains about 51.5%; butter contains 46.5 %; whale oil contains 34.0%; cream oil contains 18.7%; Oleic acid has a stable (α-type) and unstable (β-type) two types. At low temperature, it can appear as crystal; at high temperature, it appears as colorless transparent oily liquid with lard odor. It has a relative molecular mass of 282.47, relative density of 0.8905 (20 ℃ liquid), M.p. of 16.3 ° C (α), 13.4 ° C (β), boiling point of 286 °C (13.3 103 Pa), 225 to 226 °C(1.33 103 Pa), 203 to 205 °C (0.677 103 Pa), and 170 to 175 °C (0.267 103 to 0.400 103 Pa), the Refractive index of 1.4582 and viscosity of 25.6 mPa ? s (30 ° C). It is insoluble in water, being soluble in benzene and chloroform. It is miscible with methanol, ethanol, ether and carbon tetrachloride. Because of containing double bond, it can be easily subject to air oxidation, thus producing bad smell with the color turning yellow. Upon using nitrogen oxides, nitric acid, mercurous nitrate and sulfurous acid for treatment, it can be converted to elaidic acid. It can be converted into stearic acid upon hydrogenation. Double bond is easy to react with halogen to produce halogen stearic acid. It can be obtained through the hydrolysis of olive oil and lard oil, followed by steam distillation and crystallization or extraction for separation. Oleic acid is an excellent solvent for other oils, fatty acids and oil-soluble substances. It can be used for the manufacture of soap, lubricants, flotation agents, such as ointment and oleate. ? Fig. 1 the vegetable oleic acid; |
|
Determination of Unsaturation degree of Oleic Acid |
Oleic acid molecules have an unsaturated double bond with the chemical properties of single unsaturated carboxylic acid. It is capable for having addition reaction bromine. Add 2 mL of CCl4 and 0.5 mL of oleic acid to a test tube and mix. Take another test tube, put into 1 mL of CCl4, add 1 drop of bromine and shake uniformly. The CCl4 of bromine solution is dropped into the CCl4 solution of oleic acid; shake the tube and the color of bromine will recede: C17H33COOH + Br2 → C17H33Br2COOH. |
|
Oleic acid and linoleic acid |
Oleic acid, linoleic acid both belong to unsaturated long-chain fatty acids. Their molecules respectively contain one and two double bonds. In addition to providing animal energy, it is also indispensable nutrients. As they can’t be synthesized through fat and carbohydrate inside the animal body, it is thus called essential fatty acids. Some animals can produce linoleic acid from arachidonic acid. These two kinds of acids are mostly existed in the vegetable oil so poultry and pigs intake of vegetable oil are not lacking of it. Recently, however, a requirement for linoleic acid has been appeared in the recently poultry feeding standards because linoleic acid will finally generate EPA (eicosapentaenoic acid) in the body metabolism. EPA is the n-6 series of fatty acids and plays an important physiological function in the body. It is the component of phospholipids composed of cell membrane. These metabolic end products started from the linoleic acid are mostly contained in fish oil. Feeding with egg chickens with fish oil containing high content of EPA, DHA can produce eggs which can reduce cholesterol after being eaten by human. |
|
Chemical properties |
It appears as colorless to pale yellow oily liquid. It is insoluble in water but soluble in benzene, chloroform and is miscible with alcohol and ether. |
|
Benefits |
Oleic acid is a fatty acid found in animal and vegetable oils. Oleic acid is a mono-saturated fat generally believed to be good for one's health. Indeed, it is the chief fatty acid found in olive oil, comprising 55 to 85 percent of the important substance, which is commonly used in Mediterranean cuisine and has been hailed for its therapeutic characteristics since antiquity. Modern studies support the notion of the benefits of consuming olive oil, since evidence suggests that oleic acid helps lower levels of harmful low-density lipoproteins (LDLs) in the bloodstream, while leaving levels of beneficial high-density lipoproteins (HDLs) unchanged. Found also in significant quantities in canola, cod liver, coconut, soybean, and almond oils, oleic acid can be consumed from a variety of sources, some of which may soon contain even higher levels of the valuable fatty acid due to the efforts of genetic engineers. Oleic acid occurs naturally in greater quantities than any other fatty acid. It is present as glycerides in most fats and oils. High concentrations of oleic acid can lower blood levels of cholesterol. It is used in the food industry to make synthetic butters and cheeses. It is also used to flavor baked goods, candy, ice cream, and sodas. According to the American Diabetes Association, more than 25 million Americans have diabetes. In addition, 7 million have undiagnosed diabetes, and 79 million others have prediabetes. In a study published in February 2000 in the medical journal "QJM," researchers in Ireland found that diets rich in oleic acid improved the participants' fasting plasma glucose, insulin sensitivity and blood circulation. Lower fasting glucose and insulin levels, along with enhanced blood flow, suggest better diabetes control and less risk for other diseases. For millions of people with diagnosed diabetes and prediabetes, consuming foods rich in oleic acid may be beneficial in controlling the disease. |
|
Preparation |
(1) extract oleic acid directly from the vegetable oil, namely, apply saponification for extraction, upon stirring, send the oil into the steam to make the temperature rise to 80~100 °C, then add alkaline solution to hydrolyze the oil fat. After hydrolysis, we can obtain mixed fatty acids. Further apply distillation and cooling so that they are separated. This method demands large labor intensity, energy consumption, alkali consumption and is generally not used. (2) Take vegetable oil or animal oil as raw material; apply atmospheric catalytic hydrolysis for preparation of oleic acid. For the catalyst, we can also choose alkyl benzene sulfonic acid. Alternatively, we can apply intermittent pressure catalytic cracking method using zinc oxide as the catalyst at a pressure of 10.13 × 105~35.46 × 105 Pa and temperature of 150~230 °C. We can also apply continuous, backwash and high pressure lysis under the pressure of 5~5.2MPa and temperature of 260 ℃. For the catalyst, we can also use zinc oxide. This method can produce higher efficiency according to the previous two kinds, but being not suitable for oil fat of higher unsaturated degree and high content of hydroxy. Using the above three methods, we can prepare mixed fatty acids, and then conduct separation and refinement. First apply distillation for crude fraction, and the distillation was carried out under reduced pressure (0.133 103 to 1.07 103 Pa). Maintain the distillation temperature not exceed 260 °C. The distilled fatty acid is further subject to rectification using the difference between the boiling points of the fatty acids. We can also conduct refinement using crystallization method based on the melting points of various kinds of fatty acids. We can also apply solvent extraction for refining. (3) Synthetic oleic acid. In 1925, people had already used ethyl acetoacetate as raw material for the synthesis of oleic acid. With the development of petrochemical industry, synthetic oleic acid process has also been developed. We can prepare oleic acid from petroleum olefin. |
|
Toxicity |
It is natural fatty acids, being non-toxic. It can be safely used in food (FDA, §172.862, 2000). LD50: 74 g/kg (rat, oral). |
|
Usage limitation |
FEMA (mg/kg): soft drinks 0.25 to 0.40, cold drinks 30, candy 3.5, baked food 25, seasoning 0.02. |
|
Production method |
Oleic acid and other fatty acids together, are presented in all kinds of animal and vegetable oil fats in the form of glycerides. In animal fats, oleic acid can account for about 40-50% of the fatty acids. Its content in the vegetable oil can vary largely with the content in tea oil being as high as 83%, being 54% in peanut oil while the coconut oil only contains about 5-6%. Oleic acid is the co-product upon the production of stearic acid. The industrial stearic acid and industrial oleic acid actually both contain other fatty acids. There are many oil fats raw materials used for the production of stearic acid and oleic acid. The industry generally take mixed fat formulations, such as 30% melting beef tallow, 10% melting lard, 40% of bone oil and 20% of cottonseed oil. In the mixed fatty acid obtained through refinement and hydrolysis of oil fat, the difference of the melting point between the saturated and unsaturated acid is large. The yield of stearic acid and oleic acid depends mainly on the oil ester formula. Under normal circumstances, cold compressing can give 30-50% oleic acid and 50-70% stearic acid. Put the animal and vegetable oils and emulsions to hydrolysis at 105 ℃; remove the stearic acid after one step of compressing. Separate out the crude oleic acid and conduct dehydration, distillation and freezing; then conduct the second time compressing to remove palmitic acid, and finally obtain the finished product through refinement and dehydration. This method can be applied for co-production of stearic acid. For the same logic, use oleic acid for production of stearic acid will also produce oleic acid. Fixed consumption amount of raw materials: animal and vegetable oils and fats: 1950 kg/t, sulfuric acid (98%) 210kg/t. Use oil fat containing a certain amount of oleic acid as raw materials, for example, tallow, lard, palm oil and hydrolyze out the fatty acids. Use solvent to dissolve fatty acids and cool it to remove solid fatty acids and obtain the crude oleic acid. Then further dissolve it in the solvent, cooling at low temperature to crystallize the oleic acid out. |
|
Definition |
ChEBI: An octadec-9-enoic acid in which the double bond at C-9 has Z (cis) stereochemistry. |
|
Production Methods |
Oleic acid is obtained by the hydrolysis of various animal and vegetable fats or oils, such as olive oil, followed by separation of the liquid acids. It consists chiefly of (Ζ)-9-octadecenoic acid. Oleic acid that is to be used systemically should be prepared from edible sources. |
|
General Description |
Colorless to pale yellow liquid with a mild odor. Floats on water. |
|
Air & Water Reactions |
Keep cis-9-Octadecenoic acid well closed; protect cis-9-Octadecenoic acid from air and light. . May form peroxides upon exposure to air. This is taken to account for an explosion that occurred, by the mixing of the acid with aluminum, [J. Chem. Educ., 1956, 36, 308]. Water Insoluble. |
|
Reactivity Profile |
cis-9-Octadecenoic acid is a carboxylic acid. Carboxylic acids donate hydrogen ions if a base is present to accept them. They react in this way with all bases, both organic (for example, the amines) and inorganic. Their reactions with bases, called "neutralizations", are accompanied by the evolution of substantial amounts of heat. Neutralization between an acid and a base produces water plus a salt. Carboxylic acids with six or fewer carbon atoms are freely or moderately soluble in water; those with more than six carbons are slightly soluble in water. Soluble carboxylic acid dissociate to an extent in water to yield hydrogen ions. The pH of solutions of carboxylic acids is therefore less than 7.0. Many insoluble carboxylic acids react rapidly with aqueous solutions containing a chemical base and dissolve as the neutralization generates a soluble salt. Carboxylic acids in aqueous solution and liquid or molten carboxylic acids can react with active metals to form gaseous hydrogen and a metal salt. Such reactions occur in principle for solid carboxylic acids as well, but are slow if the solid acid remains dry. Even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in cis-9-Octadecenoic acid to corrode or dissolve iron, steel, and aluminum parts and containers. Carboxylic acids, like other acids, react with cyanide salts to generate gaseous hydrogen cyanide. The reaction is slower for dry, solid carboxylic acids. Insoluble carboxylic acids react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by the reaction of carboxylic acids with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. Carboxylic acids, especially in aqueous solution, also react with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Their reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Like other organic compounds, carboxylic acids can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. A wide variety of products is possible. Like other acids, carboxylic acids may initiate polymerization reactions; like other acids, they often catalyze (increase the rate of) chemical reactions. |
|
Health Hazard |
Industrial use of compound involves no known hazards. Ingestion causes mild irritation of mouth and stomach. Contact with eyes or skin causes mild irritation. |
|
Fire Hazard |
cis-9-Octadecenoic acid is combustible. |
|
Pharmaceutical Applications |
Oleic acid is used as an emulsifying agent in foods and topical pharmaceutical formulations. It has also been used as a penetration enhancer in transdermal formulations,to improve the bioavailability of poorly water-soluble drugs in tablet formulations, and as part of a vehicle in soft gelatin capsules, in topical microemulsion formulations,in oral self-emulsifying drug delivery systems,in oral mucoadhesive patches,and in a metered dose inhaler.Oleic acid was shown to be an important factor in the hypoglycemic effect produced by multiple emulsions containing insulin intended for intestinal delivery of insulin. The phase behavior of sonicated dispersions of oleic acid has been described,and mechanisms for the topical penetrationenhancing actions of oleic acid have been presented. Oleic acid has been reported to act as an ileal ‘brake’ that slows down the transit of luminal contents through the distal portion of the small bowel. Oleic acid labeled with 131I and 3H is used in medical imaging. |
|
Biochem/physiol Actions |
Oleic acid is a colourless, odourless fatty acid that blocks the glucose production and food intake when administered intracerebroventricularly. |
|
Safety Profile |
Poison by intravenous route. Mildly toxic by ingestion. Mutation data reported. A human skin and eye irritant. Questionable carcinogen with experimental tumorigenic data. Combustible when exposed to heat or flame. To fight fire, use CO2, dry chemical. The peroxidzed acid explodes on contact with aluminum. Potentially dangerous reaction with perchloric acid + heat. When heated to decomposition it emits acrid smoke and irritating fumes. |
|
Safety |
Oleic acid is used in oral and topical pharmaceutical formulations. In vitro tests have shown that oleic acid causes rupture of red blood cells (hemolysis), and intravenous injection or ingestion of a large quantity of oleic acid can therefore be harmful. The effects of oleic acid on alveolar and buccal epithelial cells in vitro have also been studied; the in vitro and in vivo effects of oleic acid on rat skin have been reported. Oleic acid is a moderate skin irritant; it should not be used in eye preparations. An acceptable daily intake for the calcium, sodium, and potassium salts of oleic acid was not specified by the WHO since the total daily intake of these materials in foods was such that they did not pose a hazard to health. LD50 (mouse, IV): 0.23 g/kg LD50 (rat, IV): 2.4 mg/kg LD50 (rat, oral): 74 g/kg |
|
Carcinogenicity |
Some recent studies suggested that oleic acid may decrease the incidence of mammary gland tumors of some rodent species. In a reviewof several fatty acids, Ip concludes that there is little evidence for the protective effect of oleic acid on the development of cancer. |
|
storage |
On exposure to air, oleic acid gradually absorbs oxygen, darkens in color, and develops a more pronounced odor. At atmospheric pressure, it decomposes when heated at 80–100°C. Oleic acid should be stored in a well-filled, well-closed container, protected from light, in a cool, dry place. |
|
Purification Methods |
Purify the acid by fractional crystallisation from its melt, followed by molecular distillation at 10 -3mm, or by conversion to its methyl ester, the free acid can be crystallised from acetone at -40o to -45o (12mL/g). For purification by the use of lead and lithium salts, see Keffler and McLean [J Soc Chem Ind (London) 54 176T 1935]. Purification based on direct crystallisation from acetone is described by Brown and Shinowara [J Am Chem Soc 59 6 1937, pK White J Am Chem Soc 72 1857 1950]. [Beilstein 2 H 463, 2 I 198, 2 II 429, 2 III 1387, 2 IV 1641.] |
|
Incompatibilities |
Incompatible with aluminum, calcium, heavy metals, iodine solutions, perchloric acid, and oxidizing agents. Oleic acid reacts with alkalis to form soaps. |
|
Regulatory Status |
GRAS listed. Included in the FDA Inactive Ingredients Database (inhalation and nasal aerosols, tablets, topical and transdermal preparations). Included in nonparenteral medicines (metered dose inhalers; oral capsules; oral prolonged release granules; topical creams and gels) licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients. |
InChI:InChI=1/C18H34O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20/h9-10H,2-8,11-17H2,1H3,(H,19,20)/b10-9-
112-80-1 Relevant articles
Isoflavone glycosides from aerial parts of Artemisia absinthium
Ahamad,Naquvi,Ali,Mir
, p. 996 - 1000 (2014)
Two new isoflavone glycosides, designate...
Chloroacetoxylation of oleic acid - a kinetic study
Doulia, Danae,Rigas, Fotis,Gimouhopoulos, Kostantinos
, p. 239 - 242 (2000)
The kinetics of the addition reaction of...
Hydrothermal deoxygenation of triglycerides over Pd/C aided by in situ hydrogen production from glycerol reforming
Hollak, Stefan A. W.,Ari?ns, Maxim A.,De Jong, Krijn P.,Van Es, Daan S.
, p. 1057 - 1062 (2014)
A one-pot catalytic hydrolysis-deoxygena...
-
Walborsky et al.
, p. 2590,2592 (1951)
-
Kinetics and pathways for an algal phospholipid (1,2-dioleoyl-sn-glycero-3- phosphocholine) in high-temperature (175-350 °c) water
Changi, Shujauddin,Savage, Phillip E.,Matzger, Adam J.
, p. 2856 - 2867,12 (2012)
We examined the behavior of 1,2-dioleoyl...
-
Adkins,Billica
, p. 695,696 (1948)
-
-
Jensen et al.
, p. 580 (1970)
-
Lipase mimetic cyclodextrins
Lee, Youngjun,Devaraj, Neal K.
, p. 1090 - 1094 (2021)
Glycerophospholipids (GPLs) perform nume...
Radical nitrile transfer with methanesulfonyl cyanide or P-toluenesulfonyl cyanide to carbon radicals generated from the acyl derivatives of N-hydroxy-2-thiopyridone
Barton,Jaszberenyi,Theodorakis
, p. 3321 - 3324 (1991)
Reaction of methanesulfonyl cyanide or p...
Phospholipases a1 from armillaria ostoyae provide insight into the substrate recognition of a/b-hydrolase fold enzymes
Dippe, Martin,Mueller, Mathias Q.,Sinz, Andrea,Ulbrich-Hofmann, Renate
, p. 1435 - 1448 (2012)
Four enzymes with phospholipase A1(PLA1)...
Synthesis and properties of ascorbyl esters catalyzed by lipozyme TL im using triglycerides as acyl donors
Reyes-Duarte,Lopez-Cortes,Torres,Comelles,Parra,Pena,Ugidos,Ballesteros,Plou
, p. 57 - 64 (2011)
Esters of l-ascorbic acid with long-chai...
Chemical constituents from the antitumor fraction of trachyrhamphus serratus
Wang, Mengyue,He, Yunjin,Nie, Yuxiao,Li, Xiaobo
, p. 465 - 466 (2011)
-
Purification and biochemical characterization of an extracellular lipase from Pseudomonas fluorescens MTCC 2421
Chakraborty, Kajal,Paulraj
, p. 3859 - 3866 (2009)
An extracellular lipase produced by Pseu...
Fatty acid eutectic mixtures and derivatives from non-edible animal fat as phase change materials
Gallart-Sirvent, Pau,Martín, Marc,Villorbina, Gemma,Balcells, Mercè,Solé, Aran,Barrenche, Camila,Cabeza, Luisa F.,Canela-Garayoa, Ramon
, p. 24133 - 24139 (2017)
A set of compounds from non-edible fat w...
Improving the activity and stability of Yarrowia lipolytica lipase Lip2 by immobilization on polyethyleneimine-coated polyurethane foam
Cui, Caixia,Tao, Yifeng,Li, Lingli,Chen, Biqiang,Tan, Tianwei
, p. 59 - 66 (2013)
In this study, polyurethane foam (PUF) w...
Antiparasitic Ovalicin Derivatives from Pseudallescheria boydii, a Mutualistic Fungus of French Guiana Termites
Elie, Nicolas,Eparvier, Véronique,Grayfer, Tatyana,Grellier, Philippe,Hebra, Téo,Leman-Loubière, Charlotte,Sorres, Jonathan,Stien, Didier,Touboul, David
, (2022/02/19)
Social insects are in mutualism with mic...
Oxidation of Primary Alcohols and Aldehydes to Carboxylic Acids via Hydrogen Atom Transfer
Tan, Wen-Yun,Lu, Yi,Zhao, Jing-Feng,Chen, Wen,Zhang, Hongbin
supporting information, p. 6648 - 6653 (2021/09/08)
The oxidation of primary alcohols and al...
Biochemical and biophysical characterisation of a small purified lipase from Rhizopus oryzae ZAC3
Ayinla, Zainab A.,Ademakinwa, Adedeji N.,Gross, Richard A.,Agboola, Femi K.
, (2021/02/16)
The characteristics of a purified lipase...
112-80-1 Process route
-
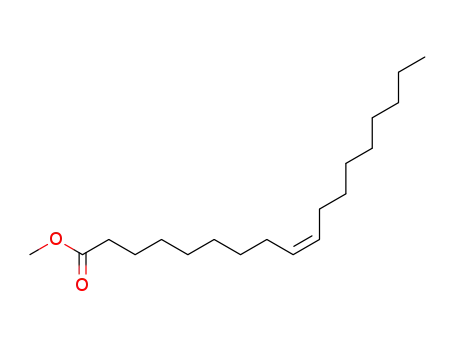
-
112-62-9
Methyl oleate

-
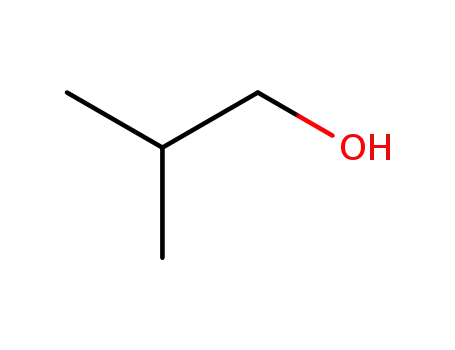
-
78-83-1
2-methyl-propan-1-ol

-
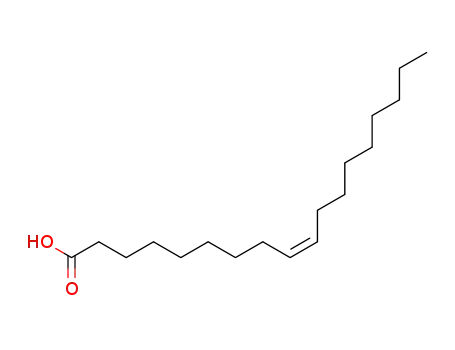
-
112-80-1,2027-47-6
cis-Octadecenoic acid

-
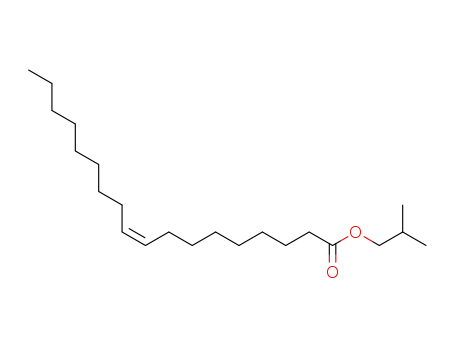
-
10024-47-2
Priolube 1414
| Conditions | Yield |
|---|---|
|
With
lipase/acyltransferase from Candida albicans; water;
In
aq. phosphate buffer;
at 30 ℃;
pH=6.5;
Reagent/catalyst;
Catalytic behavior;
Enzymatic reaction;
|
-
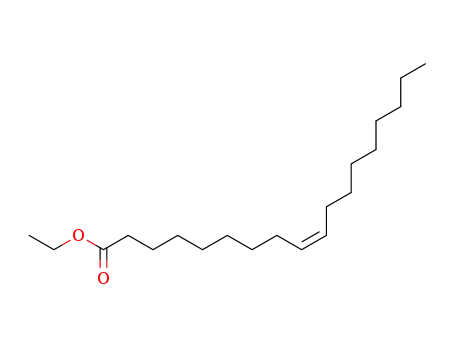
-
111-62-6
oleic acid ethyl ester

-

-
112-80-1,2027-47-6
cis-Octadecenoic acid

-

-
10024-47-2
Priolube 1414
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 2 steps
1: water; lipase/acyltransferase from Candida dubliniensis / aq. phosphate buffer / 30 °C / pH 6.5 / Enzymatic reaction
2: water; lipase/acyltransferase from Candida albicans / aq. phosphate buffer / 30 °C / pH 6.5 / Enzymatic reaction
With
lipase/acyltransferase from Candida albicans; lipase/acyltransferase from Candida dubliniensis; water;
In
aq. phosphate buffer;
|
112-80-1 Upstream products
-
60-29-7
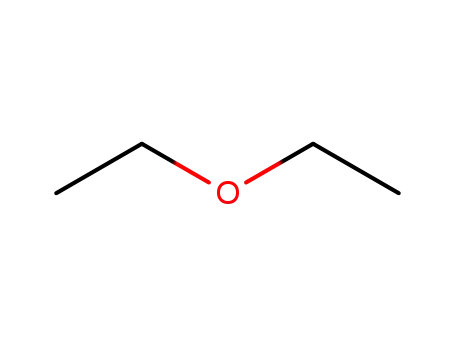
diethyl ether
-
67-66-3
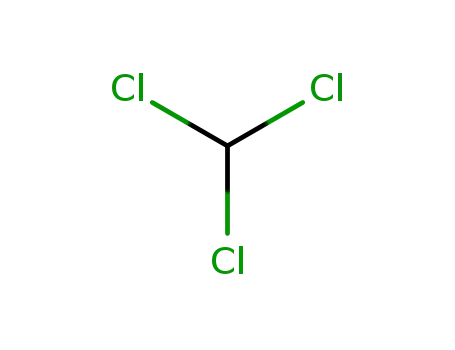
chloroform
-
56554-80-4

1-chloro-heptadec-8-ene
-
143-33-9
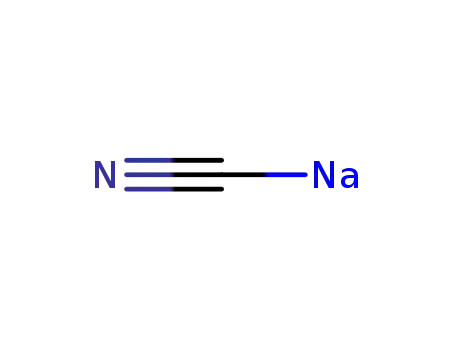
sodium cyanide
112-80-1 Downstream products
-
112-62-9

Methyl oleate
-
3971-54-8
cis-9,10-Methylen-octadecansaeure-methylester
-
21656-45-1
2-heptadec-8c-enyl-4,5-dihydro-1H-imidazole
-
111-59-1
propyl oleate
Relevant Products
-
Tesamorelin
CAS:218949-48-5
-
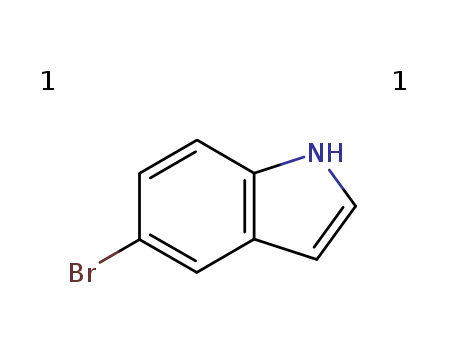
5-Bromoindole
CAS:10075-50-0
-
Aluminum chlorohydrate
CAS:1327-41-9